NEET Previous Year Questions (2014-2024): Surface Chemistry (Old NCERT) | Chemistry Class 12 PDF Download
Q.1. Which one is an example of heterogenous catalysis? (2023)
A: Oxidation of sulphur dioxide into sulphur trioxide in the presence of oxides of nitrogen
B: Hydrolysis of sugar catalysed by H+ ions
C: Decomposition of ozone in presence of nitrogen monoxide
D: Combination between dinitrogen and dihydrogen to form ammonia in the presence of finely divided iron
Ans: D
Combination of N2 and H2 to form NH3 in presence of finely divided Fe is an example of heterogeneous catalysis.
All other are examples of homogeneous catalysis.

Q.2. Pumice stone is an example of (2023)
A: Sol
B: Gel
C: Solid sol
D: Foam
Ans: C
Pumice stone is a solid sol.
Dispersed phase: Gas
Dispersed medium: Solid
Q.3. The incorrect statement regarding enzymes is: (2022)
A: Enzymes are polysaccharides.
B: Enzymes are very specific for a particular reaction and substrate.
C: Enzymes are biocatalysts.
D: Like chemical catalysts enzymes reduce the activation energy of bio processes.
Ans: A
Enzymes are proteins.
Q.4. Given below are two statements
Statement I: In the coagulation of a negative sol, the flocculating power of the three given ions is in the order Al3+ > Ba2+ > Na+
Statement II: In the coagulation of a positive sol, the flocculating power of the three given salts is in the order NaCl > Na2SO4 > Na3PO4
In the light of the above statements, choose the most appropriate answer from the options given below (2022)
A: Statement I is correct but Statement II is incorrect
B: Statement I is incorrect but statement II is correct
C: Both Statement I and Statement II are correct
D: Both Statement I and Statement II are incorrect
Ans: A
Statement – II is incorrect. The flocculation power of anions is Na3PO4 > Na2SO4 > NaCl
Q.5. The right option for the statement "Tyndall effect is exhibited by", is (2021)
A: Starch solution
B: Urea solution
C: NaCl solution
D: Glucose solution
Ans: A
Tyndall effect is exhibited by colloidal solutions. Starch solution is a colloidal solution.
Q.6. Measuring Zeta potential is useful in determining which property of colloidal solution? (2020)
A: Stability of the colloidal particles
B: Size of the colloidal particles
C: Viscosity
D: Solubility
Ans: A
A large +ve or –ve value of Zeta potential indicate good stability of the colloidal particles.
Q.7. Which mixture of the solutions will lead to the formation of negatively charged colloidal [Agl]l– solution ? (2019)
A: 50 mL of 1 M AgNO3 + 50 mL of 1.5 M KI
B: 50 mL of 1 M AgNO3 + 50 mL of 2 M KI
C: 50 mL of 2 M AgNO3 + 50 mL of 1.5 M KI
D: 50 mL of 0.1 M AgNO3 + 50 mL of 0.1 M KI
Ans: B
Generally charge present on the colloid is due to adsorption of common ion from dispersion medium. Millimole of KI is maximum in option (B) (50 × 2 = 100) so act as solvent and anion I– is adsorbed by the colloid AgI formed.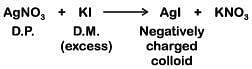
Q.8. On which of the following properties does coagulating power of an ion depend ? (2018)
A: The magnitude of the charge on the ion alone
B: Size of the ion alone
C: Both magnitude and sign of the charge the ion
D: The sign of charge on the ion alone
Ans: C
According to Hardy Schulze rule : The coagulating power of an ion depends on both magnitude and sign of the charge of the ion.
Q.9. Which one of the following characteristics is associated with adsorption ? (2016)
A: ΔG and ΔS are negative but ΔH is positive
B: ΔG is negative but ΔH and ΔS are positive
C: ΔG, ΔH and ΔS all are negative
D: ΔG and ΔH are negative but ΔS is positive
Ans: C
According to Gibbs Helmholtz equation, ΔG = ΔH & TΔS
Adsorption is a spontaneous process (where ΔS < 0, ΔG < 0 and ΔH < 0)
Q.10. Fog is a Colloidal solution of : (2016)
A: Gas in gas
B: Liquid in gas
C: Gas in liquid
D: Solid in gas
Ans: B
Q.11. Which property of colloidal solution is independent of charge on the colloidal particles ? (2015)
A: Tyndall effect
B: Coagulation
C: Electrophoresis
D: Electro-osmosis
Ans: A
Tyndall effect is independent of charge on the colloidal particles. It is an optical property and depends on the size of the colloidal particles. The Tyndall effect, also known as Tyndall scattering, is light scattering by particles in a colloid or particles in a fine suspension. However, electro-osmosis, coagulation and electrophoresis depends on the charge on the colloidal particles. These are electrical properties.
Q.12. Which of the following statements is correct for the spontaneous adsorption of a gas? (2014)
A: ΔS is positive and, therefore, ΔH should be negative
B: ΔS is positive and, therefore, ΔH should also be highly positive
C: ΔS is negative and, therefore, ΔH should be highly positive
D: ΔS is negative and therefore, ΔH should be highly negative
Ans: D
Using Gibb's-Helmholtz equation,
ΔG = ΔH - TΔS
During adsorption of a gas, entropy decreases i.e. ΔS < 0
For spontaneous adsorption, ΔG should be negative, which is possible when ΔH is highly negative.
Q.13. Which property of colloids is not dependent on the charge on colloidal particles? (2014)
A: Electro−osmosis
B: Tyndall effect
C: Coagulation
D: Electrophoresis
Ans: B
Tyndall effect is independent of charge on the colloidal particles. It is an optical property and depends on the size of the colloidal particles. The Tyndall effect, also known as Tyndall scattering, is light scattering by particles in a colloid or particles in a fine suspension. However, electro-osmosis, coagulation and electrophoresis depends on the charge on the colloidal particles. These are electrical properties.
|
108 videos|286 docs|123 tests
|

|
Explore Courses for NEET exam
|

|

















