Properties of Soil: Physical, Chemical & Biological - 2 | Agriculture Optional for UPSC PDF Download
Chemical properties of soil
The chemical characteristics of soils are influenced by the following factors:
- Inorganic Components in the Soil: The mineral content within the soil is a primary factor that distinguishes different soil types due to its prevalence and abundance in the soil.
- Organic Components in the Soil: Despite their relatively small quantities, organic materials in the soil play a significant role in determining soil fertility.
- Colloidal Properties of the Soil: Soil colloids primarily come in two forms:
- Clay Colloids: These are crucial for the absorption of a substantial amount of water.
- Organic Colloids: These aid in enhancing the soil's capacity to retain moisture and nutrients.
- Soil pH: The soil's pH value is an indicator of its chemical reactivity, determining whether the soil is acidic or alkaline in nature.
Acidity & Alkalinity
A crucial aspect of soil chemistry revolves around the soil's level of acidity, alkalinity (basicity), or neutrality.
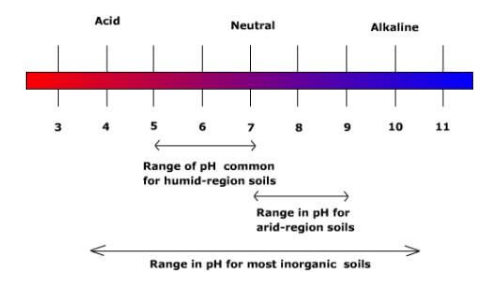
Soil pH values provide insights into its acidity, where low pH values indicate soil's acidity, while high pH values point to alkaline conditions. Most complex plants thrive in soils with pH levels ranging between 4 and 10, but the optimum pH may vary depending on the plant species.
- In arid and semi-arid regions, soils tend to be alkaline, while soils in humid regions often lean towards acidity.
- To rectify soil alkalinity and enhance soil productivity, a method involves flushing the soil with irrigation water.
- Strongly acidic soils can also hinder plant growth, but acidity can usually be corrected by introducing lime to the soil.
 The acidity or alkalinity of soil has a significant impact on ion solubility, which subsequently influences microbial and plant growth. A pH range of 6.0 to 6.8 is considered ideal for most crops because it aligns with the optimum solubility of key plant nutrients.
The acidity or alkalinity of soil has a significant impact on ion solubility, which subsequently influences microbial and plant growth. A pH range of 6.0 to 6.8 is considered ideal for most crops because it aligns with the optimum solubility of key plant nutrients.
Some minor elements, such as iron, and most heavy metals, are more soluble at lower pH levels, underscoring the importance of pH management in controlling the mobility of heavy metals in soil, which can potentially lead to groundwater contamination.
The lime requirement, or the quantity of liming material needed to elevate soil pH to a specific level, increases with CEC (cation exchange capacity). To reduce soil pH, sulfur can be introduced, which generates sulfuric acid.
Soil Colloids
Soil colloids represent the most active component within the soil and play a crucial role because their surfaces have an affinity for soil nutrients that are present in the form of positively charged mineral ions or cations in the soil solution.
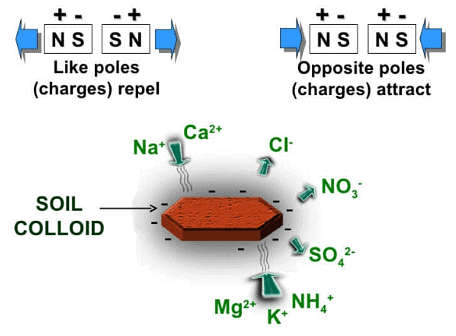 Certain cations, such as calcium (Ca++), magnesium (Mg++), potassium (K+), and sodium (Na+), are essential for plant growth. For these cations to become available to plants, they need to be in a dissolved state within the soil-water solution and in proximity to root membranes.
Certain cations, such as calcium (Ca++), magnesium (Mg++), potassium (K+), and sodium (Na+), are essential for plant growth. For these cations to become available to plants, they need to be in a dissolved state within the soil-water solution and in proximity to root membranes.
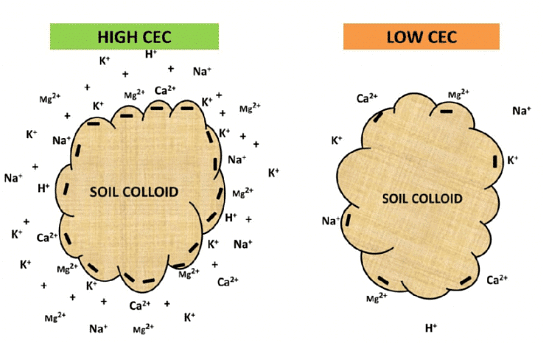
The fertility of the soil-water solution for plant utilization depends on the soil's capacity to retain and exchange these cations, a quality referred to as cation-exchange capacity. Soil colloids are vital in preventing the leaching of essential nutrients from the soil through the percolation of water, which would otherwise result in the loss of these nutrients carried away in streams.
Biological Properties of Soil
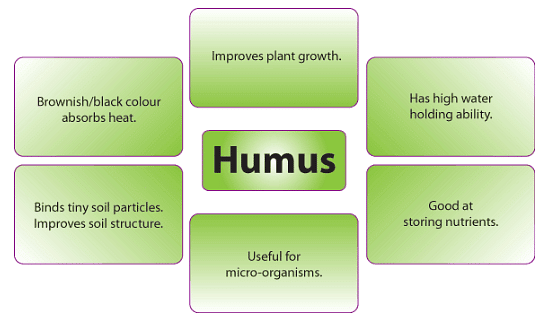
The presence of organic matter in the soil enhances soil structure and augments the soil's capacity to retain nutrients and water. Additionally, organic matter serves as a source of nourishment for soil microorganisms. Soils with low organic matter content tend to exhibit poor structure, low water retention, and are susceptible to erosion and nutrient loss through leaching, with the exception being clay soils, where clay minerals play a more significant role in determining soil structure. Conversely, soils with elevated levels of organic matter display favorable soil structure, improved water retention, and reduced risks of erosion and nutrient leaching.
Biological properties include:
- organic matter
- soil organisms
- the presence of disease-causing organisms.
Biological processes in soil formation encompass the presence and actions of both living plants and animals, along with their non-living organic byproducts.
Living plants contribute to soil formation in two primary ways.
- Biomass: This involves the generation of organic matter, encompassing the production of biomass above the soil in the form of stems and leaves, as well as within the soil through root growth. This biomass serves as the raw material for organic matter found in the O horizon and lower soil horizons. Decomposer organisms are responsible for processing this raw material, breaking it down into humus, and ultimately returning it to its initial components, carbon dioxide and water.
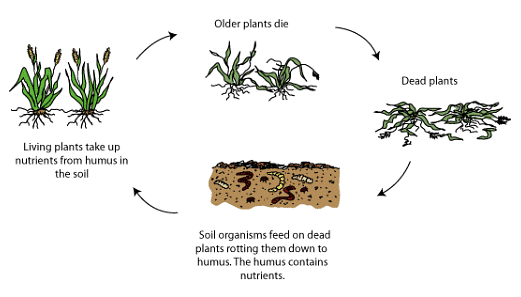
- Nutrient Recycling: This process revolves around the cycling of nutrients contained in dead plant tissues. Nutrient recycling prevents the loss of nutrients through the leaching process, wherein excess soil water moves downward through the soil.
In addition to plants, animals residing in the soil also play a significant role in the biological processes of soil formation. For instance, earthworms contribute to soil modification not only through their burrowing activities but also by passing soil through their digestive tracts.
Some of the important factors which decide the biological behavior of soil are:
- Respiration rate: CO2 evolution under standard laboratory conditions or at the field.
- Potential N/C mineralization: Increase in mineral Nitrogen or Carbon content under standard laboratory conditions.
- Earthworms: Density of earthworms.
- Bacterial biomass: Total bacterial biomass for a given soil mass.
- Bacterial diversity: It can be determined by functional groups, or describing genetic diversity.
- Presence of pathogens: By different pathology techniques, from cultures to DNA profiling.
|
52 videos|224 docs
|





















