Quality of Irrigation Water | NABARD Grade A & Grade B Preparation - Bank Exams PDF Download
Quality of Irrigation Water
Just as not all water is suitable for human consumption, the same applies to water used for plant growth. Water that contains harmful impurities for plants is considered unsuitable for irrigation. The quality of appropriate irrigation water depends significantly on the characteristics of the soil being irrigated. Water that may be detrimental for one type of soil might be acceptable or even beneficial for another.
The various impurities that render water unsuitable for irrigation are categorized as follows:
- Sediment concentration in water.
- Total soluble salt concentration in water.
- The proportion of sodium ions to other cations.
- The concentration of potentially toxic elements in water.
- Bicarbonate concentration in relation to the concentration of calcium and magnesium.
- Bacterial contamination.
Here's a breakdown of the effects of these impurities:
1. Sediment
The impact of sediment in irrigation water depends on the type of soil being irrigated. Fine sediment deposited on sandy soils can enhance fertility, but sediment from eroded areas may reduce fertility and soil permeability. In irrigation canals, sedimented water can cause siltation and increase maintenance costs. Typically, groundwater and surface water from reservoirs do not contain enough sediment to cause significant irrigation problems.
2. Total soluble salts
The presence of salts like calcium, magnesium, sodium, and potassium in irrigation water can harm plants when present in excessive amounts. These salts can reduce plant osmotic activity and impede proper aeration, leading to plant growth issues. The harmful effects of salts on plant growth depend on the concentration of salts left in the soil after irrigation. Even if the salt concentration in the irrigation water seems harmless, the salt concentration in the soil can increase to harmful levels over time as water evaporates. Therefore, the impact of salts on plant growth is largely determined by the total salt content in the soil solution.
The salt concentration in the soil solution (Cs) after the plant has used up the consumptive water (Cu) is given by the equation: Cs = CQ / [Q - (Cu - Peff)]
Where:
- Q is the quantity of water applied.
- Cu is the consumptive use of water by the plant.
- Peff is the useful rainfall.
- C is the concentration of salt in the irrigation water.
- CQ is the total salt applied to the soil with the irrigation water.
Salt concentration is typically expressed in ppm (parts per million) or mg/L (milligrams per liter), both of which are equivalent. The critical salt concentration in irrigation water depends on various factors, but concentrations exceeding 700 ppm can harm some plants, while levels over 2000 ppm are detrimental to all crops.
Salt concentration is typically assessed by measuring the electrical conductivity of water, with the two being directly related. Electrical conductivity is expressed in micro-mhos per centimeter. When the electrical conductivity value is up to 250 micro-mhos/cm at 25°C, it is categorized as low conductivity water (C1). If the value falls between 250 and 750, it is termed medium conductivity water (C2). High conductivity water (C3) encompasses values ranging from 750 to 2250, while values exceeding 2250 are classified as very high conductivity water (C4). The suitability of these four water types for irrigation purposes is discussed in Table 1.1. This classification, as presented in Table 1.1, originated from U.S.D.A. Handbook No. 60 in 1954.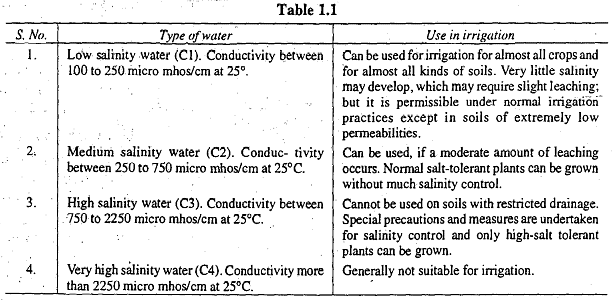
3. Sodium Ion Proportion Relative to Other Cations
In many soils, calcium and magnesium ions are predominant, with only small amounts of sodium ions. Normally, the percentage of sodium ions is less than 5% of the total exchangeable cations. When this percentage increases to around 10% or more, the soil's particle aggregation breaks down. Consequently, the soil becomes less permeable, has poor tilth, develops a crust when dry, and its pH tends to become more alkaline. Soils with high sodium content are characterized as plastic and sticky when wet, prone to forming clods, and crusting upon drying.
The proportion of sodium ions in the soil is typically quantified using a parameter known as the Sodium-Absorption Ratio (SAR), which indicates the sodium-related risks associated with water. SAR is defined as: SAR = Na+ / √(Ca²⁺ + Mg²⁺)
In this equation, ion concentrations are expressed in equivalent per million (epm). The epm value is obtained by dividing the concentration of salt in mg/l or ppm by its combining weight (i.e., atomic weight + valence).
Based on the SAR value, water can be categorized into different types:
- SAR between 0 and 10: Low Sodium Water (S1)
- SAR between 10 and 18: Medium Sodium Water (S2)
- SAR between 18 and 26: High Sodium Water (S3)
- SAR exceeding 26: Very High Sodium Water (S4)
The suitability of these four water types for irrigation is discussed in Table 1.2. The SAR value can be reduced by adding gypsum (CaSO4) to the water or soil.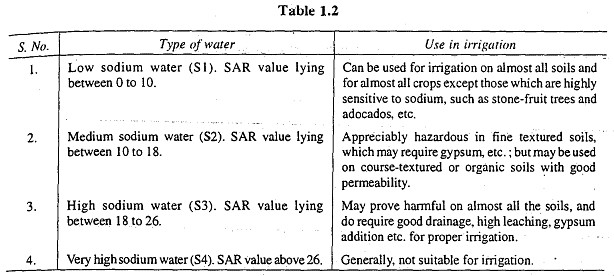
Soil classification is determined by factors such as the water's Electrical Conductivity (EC), which represents salt content, the Exchangeable Sodium Percentage (ESP) denoting the proportion of sodium in relation to the total exchangeable cations, and the soil's pH value. These criteria are used to categorize soils as either saline, alkaline, or saline-alkali, as indicated in Table 1.3 below.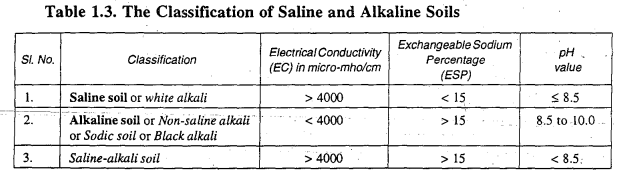
4. Presence of Potentially Harmful Elements
Numerous elements, including boron and selenium, can be harmful to plants. While small amounts of boron are essential for plant growth, concentrations exceeding 0.3 ppm can be toxic to specific plants. Levels above 0.5 ppm are particularly dangerous for certain crops like nuts, citrus fruits, and deciduous fruits. Some crops, like cotton, cereals, and certain truck crops, have moderate tolerance to boron, while others, such as dates, beets, and asparagus, are more tolerant. Even for the most tolerant crops, boron concentration should not exceed 4 ppm. Boron is commonly found in various soaps, so wastewater containing soap must be used cautiously in irrigation. Selenium, even in low concentrations, is toxic and should be avoided.
5. Bicarbonate Concentration Relative to Calcium and Magnesium
Elevated levels of bicarbonate ions can lead to the precipitation of calcium and magnesium bicarbonates from the soil solution. This can increase the proportion of sodium ions and result in sodium-related issues.
6. Bacterial Contamination
Bacterial contamination of irrigation water is typically not a serious concern, unless crops irrigated with highly contaminated water are consumed without cooking. Cash crops like cotton and nursery stock, which undergo processing after harvesting, can utilize wastewater containing some contamination without significant issues.
|
846 videos|1297 docs|420 tests
|





















