UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Raney Nickel
Raney Nickel | Chemistry Optional Notes for UPSC PDF Download
Introduction
- Raney Nickel And The Desulfurization Of Thioacetals + Hydrogenation Of Alkenes (and Alkynes)
- In a blatant plug for the Reagent Guide and the Reagents App for iPhone, each Friday I profile a different reagent that is commonly encountered in Org 1/ Org 2.

- Named reagents have a slightly mysterious air to them, conjuring up (for me, anyway) the image of a lone scientist working long hours for an elusive goal, until they finally have that “Eureka” moment. In this vein, while “Rearden metal” may solely be a work of fiction, “Raney Nickel” is very real. Raney nickel is an alloy of aluminum and nickel, which has subsequently had much of the aluminum removed through a leaching process with sodium hydroxide (NaOH). The remaining alloy has a very high surface area and also contains hydrogen gas (H2) adsorbed on the nickel surface.
Reduction Of Sulfur Groups (Dithianes) To Alkanes With Raney Nickel
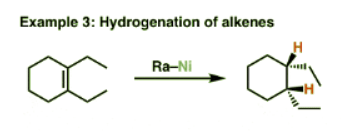
What it’s used for: Like palladium on carbon (Pd/C) and platinum on carbon (Pt/C), Raney nickel can be used for the hydrogenation of alkenes and alkynes. But what Raney nickel is used most for is its unusual property of reducing C-S bonds to C-H bonds. It’s this second application that can make this reagent uniquely useful. When combined with the formation of a thioketal from a ketone, this can serve as an alternative means of converting ketones to alkanes (just like the Wolff-Kishner reaction).
Examples

Question for Raney NickelTry yourself: Which reagent can be used to reduce C-S bonds to C-H bonds?View Solution
As A Hydrogenation Catalyst
- Here’s the second application of this reagent – as a catalyst for hydrogenation. Note that in this case we don’t necessarily need to add hydrogen gas (although it helps) – Raney nickel is usually obtained in its “activated” form, where the hydrogen is already adsorbed onto it.

- How it works: “For our purposes” (love this phrase) it’s not so important exactly how Raney nickel works. There’s something mysterious about it: the aluminum is crucial for its activity, and the metal doesn’t behave the same once it’s been completely removed. To be honest I plead ignorance on exactly how Raney nickel works its desulfurizing magic, although the catalytic hydrogenation process is likely similar to those of Pd/C and Pt/C.
- Real life tips: Perhaps a better description for Raney nickel is “Raney Mud”, because that’s what it looks and feels like. Raney nickel resembles a kind of mud or wet clay, and is actually stored in water. Determining the exact molar ratio of Raney nickel to use is also something of an art – rather than “moles”, typical procedures call for “teaspoons” [I’m not sure I’m aware of any other reagent that calls for this unit of measurement!] After dispensing (but before placing in the reaction vessel) the metal is then rinsed with water (to remove aluminum salts and ensure neutral pH). This can be something of a dicey prodedure since Raney nickel will spontaneously combust in air when traces of moisture are removed. Excess Raney nickel on benchtops, spatulas, weighing paper, etc. should be (carefully) destroyed with acid. There’s nothing like setting up your reaction and then, out of the corner of your eye, noticing little flames coming from traces of Raney nickel on your weighing paper.
Question for Raney NickelTry yourself: What is the primary use of Raney nickel?View Solution
The document Raney Nickel | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Raney Nickel - Chemistry Optional Notes for UPSC
| 1. What is the role of Raney Nickel in the reduction of sulfur groups to alkanes? |  |
Ans. Raney Nickel is used as a hydrogenation catalyst in the reduction of sulfur groups (dithianes) to alkanes. It provides a surface for the reaction to occur and promotes the transfer of hydrogen atoms to the sulfur groups, resulting in the conversion of dithianes to alkanes.
| 2. How does Raney Nickel act as a hydrogenation catalyst? |  |
Ans. Raney Nickel acts as a hydrogenation catalyst by providing a high surface area with active sites for the adsorption and activation of hydrogen molecules. This allows for the transfer of hydrogen atoms to the sulfur groups, leading to the reduction of dithianes to alkanes.
| 3. What are the advantages of using Raney Nickel for the reduction of sulfur groups? |  |
Ans. Raney Nickel offers several advantages for the reduction of sulfur groups. It is a highly efficient catalyst that can selectively reduce sulfur groups without affecting other functional groups present in the molecule. It also operates under mild reaction conditions and is readily available commercially.
| 4. Are there any limitations or drawbacks to using Raney Nickel in this reaction? |  |
Ans. While Raney Nickel is a valuable catalyst, it has some limitations. One of the drawbacks is its susceptibility to poisoning by certain substances, such as sulfur compounds or other strongly adsorbed species. This can reduce its activity and require regeneration or replacement of the catalyst.
| 5. Can Raney Nickel be used for the reduction of other functional groups apart from sulfur groups? |  |
Ans. Yes, Raney Nickel can be used for the reduction of various functional groups apart from sulfur groups. It is a versatile catalyst that can facilitate the reduction of carbonyl groups, nitro groups, and other unsaturated bonds. However, the specific reaction conditions and selectivity may vary depending on the functional group being targeted.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches