Reaction Intermediates: Definition, Examples | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Types of reaction Intermediates |

|
| Carbocation |

|
| Carbanion |

|
| Free Radicals |

|
| Carbenes |

|
| Nitrene |

|
| Benzyne |

|
| Reaction Intermediate examples |

|
Reaction intermediates are short-lived entities produced during the transformation of reactants to products during chemical reactions. Such substances are highly unstable, quickly transformed into more stable compounds, and are difficult to isolate. These properties made the reactive intermediates different than reactants and products. Reactive intermediates only appear in one of the intermediate steps, while they appear in several elementary steps in other chemical reactions. Its existence can be identified by spectroscopic methods or via chemical trapping.
Types of reaction Intermediates
Basically, reactive intermediates are categorized into 5 types:
- Carbocation
- Carbanions
- Free Radicals
- Carbenes and Nitrenes
- Benzyne
Carbocation
Carbocations are reaction intermediates that have carbon atoms containing six electrons and having a positive charge. It is also called carbonium. When a neutrally charged molecule undergoes heterolytic fission, any one of the bonded atoms (that one which possesses greater electronegativity) retains a shared pair of electrons acquires a negative charge, while the remaining atoms that do not retain electrons acquire a positive charge. This positive-charged intermediate is carbocation intermediate or carbonium ion.

Some of the features of carbocations are:
- sp2 carbocation hybridization
- Geometry of carbon atom is triagonal planar
- Highly reactive i.e. less stable
- Carbocation shows paramagnetic behaviour due to incomplete electron pairing
- Acts as electrophile
- Acts as Lewis Acid
Carbocation examples: CH3+, CH3CH2+,(CH3)2CH+, (CH3)3C+, , and so on.
Types of carbocation
Depending on the number of carbon groups attached to the carbon atom containing positive charge, carbocations are of the following types:
- Methyl Carbocation: In this types of carbocation, no any carbon group is attached to the carbon atom containing positive charge, and is the least stable carbocation.
- Primary carbocation: One carbon is attached to the carbon atom containing positive charge.
- Secondary Carbocation: Carbon containing positive charge is attached to two other carbon atom
- Tertiary Carbocation: Positively charged carbon atom is bonded to 3 other carbon atoms. It is the most stable carbocation.
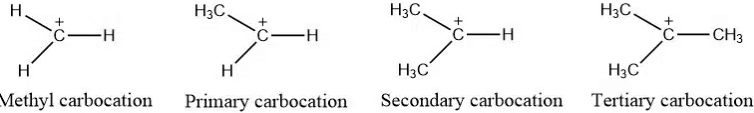
Carbocation stability:
Carbocation stability order: Methyl carbocation < primary carbocation < secondary carbocation < tertiary carbocation
Stability of carbocation
- Directly proportional to +I effect i.e. greater the number of alkyl groups, greater is the stability of carbocation. Example: CH3+< CH3CH2+<(CH3)2CH+< (CH3)3C+
- Directly proportional to hyperconjugation effect [stability = 1o < 2o < 3o carbocation
- Stability of carbocation increases by the resonance effect
- Carbocation with aromaticity possesses greater stability
Carbanion
Carbanions are reaction intermediates that have carbon atoms containing eight electrons and have a negative charge. It generally possesses 3 bonds and a lone pair of electrons. When a neutrally charged molecule undergoes heterolytic fission, any one of the bonded atoms, that one which possesses greater electronegativity retains a shared pair of electrons acquires a negative charge. This negatively charged intermediate is the carbanion intermediate.

Some of the features of carbanion are:
- sp3 carbanion hybridization
- Geometry of carbon atom is pyramidal
- Highly reactive i.e. less stable
- Carbanion shows diamagnetic behaviour due to complete electron pairing
- Acts as nucleophle
- Acts as Lewis Base
Carbanion examples: CH3–, CH3CH2–,(CH3)2CH–,(CH3)3C–, and so on.
Types of Carbanion
Depending on the number of carbon groups attached to the carbon atom containing negative charge, carbanions are of the following types:
- Methyl Carbanion: In this types of carbocation, no any carbon group is attached to the carbon atom containing negative charge, and possess most in terms of stability.
- Primary carbanion: One carbon is attached to the carbon atom containing negative charge.
- Secondary Carbanion: Carbon containing negative charge is attached to two other carbon atom
- Tertiary Carbanion: Negatively charged carbon atom is bonded to 3 other carbon atoms. It is the least stable carbocation.

Carbanion stability:
Carbanion stability order: Methyl carbanion > primary carbanion> secondary carbanion > tertiary carbanion
Stability of carbocation
- Directly proportional to -I effect i.e. lesser the number of alkyl groups, greater is the stability of carbocation. When an alkyl group is linked to the carbanions (negatively charged carbon atom), it prefers to discharge electrons toward the carbon, and destabilizes the carbanions by increasing the intensity of the negative charge on the carbon. Example: CH3–>CH3CH2–>(CH3)2CH–>(CH3)3C–
- Stability of carbocation increases by the resonance effect and the aromaticity
- Carbocation with more s-character possesses greater stability
Differences between Carbanion and Carbocation

Free Radicals
Free radical is defined as any atom or group of atoms that contain unpaired or odd electrons. In other words, free radicals are atoms or groups of atoms with unpaired electrons in outer shell configuration. When a neutral molecule undergoes homolytic fission, each of the bonded atoms retains one electron from the shared pair, and hence free radicals are produced.
 structure of free radical
structure of free radical
Some of the features of free radicals are:
- sp2 hybridization
- Geometry of carbon atom is planar, and have bond angles of 120o
- Highly reactive i.e. less stable
- Free radical shows paramagnetic behaviour due to spin of the odd electron
- Behave as oxidant or reductant as it has tendency to accept or donate electrons
Examples of free radicals
Some of the examples of free radicals are
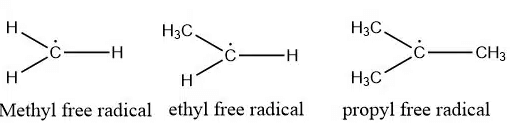
Types of free radical
Mostly there are two types of free radicals. They are neutral free radical and Charged (cation/anion) free radical.
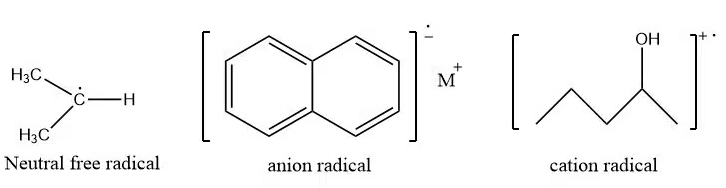
Sources of free radicals
- Environmental pollution
- Radiations
- Ozone layer
- Mitochondria
- Peroxisomes
Free radical formation
Some of the major ways of generation of free radicals include:
Homolytic cleavage:

Photocatalytic cleavage:
Abstraction of atom: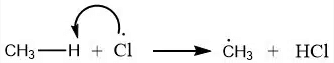
Free radical stability:
Free radical stability order: Methyl free radical < primary free radical < secondary free radical < tertiary free radical
Stability of free radical
- Greater the number of hyperconjugating structures of species, greater is the stability of free radical. Thus the increasing order of stability of free radical is 1o < 2o < 3o free radical
- Stability of free radical increases by the resonance effect
Carbenes
A carbene is a molecule containing a neutral carbon atom that possesses two bond pairs and two unshared electrons. Carbenes are short-lived reaction intermediates formed during the course of reactions.
The general formula of carbene is R-(C:)-R’ or R=C:.
Examples:
Types of carbene
Depending upon whether the two non-bonded electrons on carbene are paired or unpaired, a carbene is classified into two types viz. singlet and triplet carbene.
Singlet Carbene
The unshared electrons are paired in singlet carbene and represented as follows:
Triplet carbene
The unshared electrons are unpaired in triplet carbene and represented as follows: Triplet carbenes are also called diradicals.
Triplet carbenes are also called diradicals.
Structure of Carbene
Hybridization of Singlet Carbene
 hybridization in singlet carbene
hybridization in singlet carbene
Singlet carbenes have their carbon in a sp2 hybridized state. Of the three sp2 hybrid orbitals, two are used in forming two single bonds with monovalent atoms or groups attached to the carbon. The unshared pair of electrons are present in the third sp2 hybrid orbital and the unhybridized p-orbital is empty.
 structure of singlet carbene
structure of singlet carbene
Hybridization of Triplet carbene
 hybridization of triplet carbene
hybridization of triplet carbene
Triplet carbene has its carbon in a sp hybridized state. The two sp hybrid orbitals form two bonds with monovalent atoms or groups attached to the carbon. The two unhybridized p-orbitals contain one electron each.
 structure of triplet carbene
structure of triplet carbene
Stable carbenes
Two non-bonded electrons are present in the same orbitals in singlet carbene. As a result, electronic repulsion takes place. Triplet carbene is lower in energy and more stable.
Carbene Reactions
Addition Reaction
Carbene adds to an alkene to produce cyclopropane derivatives. The addition of singlet carbene to an alkene is stereospecific.
Insertion Reaction
The most reactive species, singlet carbene can insert itself between the carbon and hydrogen bond. For example, the insertion of (:CH2) into propane would result into a mixture of n-butane and iso-butane.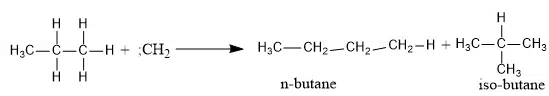
Rearrangement Reaction
Carbene may undergo rearrangement with the migration of alkyl or hydrogen to form stable molecules.
Nitrene
Nitrenes are electron deficient and reactive intermediates, in which the structure of nitrene reveals that there are six electrons around nitrogen. These are nitrogen analogs of carbene and hence something referred to as azo carbene.

Nitrene Hybridization
Nitrenes are nitrogen analogues of carbenes. The nitrogen atom possesses only six electrons; in nitrenes, the triplet state is lower in energy than the singlet state.
Nitrene Examples

Singlet and triplet nitrenes
Singlet nitrene is a kind of nitrene in which unshared electrons are paired. It is represented as:

Singlet nitrene is less stable form.
Triplet nitrene is a nitrene in which unshared electrons are not paired. It is represented as:
Triplet nitrene is thus free radical.
Generation/ Formation of Nitrene
By the reduction of nitro compound with trialkoxy phosphite
By α-elimination reaction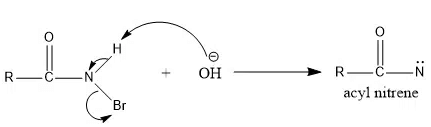
Reactions of Nitrene
Addition to C=C double bond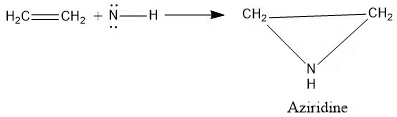
The addition of singlet nitrene is stereospecific.
Rearrangement reaction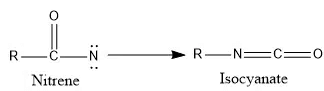
Dimerization

Azobenzene is often obtained by the dimerization of aryl nitrenes.
Benzyne
Benzyne or arynes are highly reactive reaction intermediates that are derived formally by the removal of two adjacent substituents from aromatic rings, leaving behind two electrons to be distributed between two orbitals.
Benzyne lewis structure
Benzyne is usually represented as a single molecule with a carbon-carbon triple bond. This strained π-bond is formed by the lateral overlap of the two orbitals in the plane of the ring. In fact, one π bond is normal and is just part of the aromatic system. The new π bond formed by the overlap of two sp2 orbitals outside the ring is abnormal and quite strained. This external π bond is very weak, which is why benzyne is a very unstable and highly reactive intermediate.
 Structure of Benzyne intermediate
Structure of Benzyne intermediate
Benzyne Precursor
The precursor to benzyne is benzene diazonium-2-carboxylate which decomposes to give benzyne as intermediate.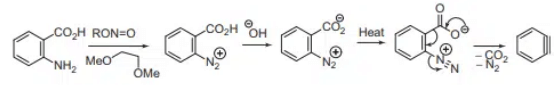 Benzyne precursor
Benzyne precursor
Benzyne formation mechanism/Generation of Benzyne intermediate
Some of the common methods of Benznye formation are shown below in the figure. Benzyne formation
Benzyne formation
Bromobenzene is treated with a strong base like sodium amide to generate benzyne intermediate, which reacts with ammonia solution to give aniline.
Mechanism
Formation of benzyne intermediate: Base abstracts a proton from ortho position to form carbanion which losses bromine atom to generate benzyne intermediate.
Formation of the final product: Benzyne reacts with ammonia to form aniline. There may occur either ipso or cine substitution.
Benzyne properties
- Benzyne shows the properties of a highly reactive alkyne, participating in a range of cycloaddition reactions.
- benzyne resembles a carbene, having a similar electronic arrangement of two electrons distributed between two orbitals and behaving as a powerful electrophile.
- Benzyne is electron-deficient and will be attacked by nucleophiles in a reaction that opens the π bond not part of the aromatic cloud, and produces a new carbanion. Protonation completes the sequence to give the aromatic substitution product.
Benzyne dimerization
The dimerization of benzyne intermediate takes place as shown in the following figure.
 Benzyne dimerization
Benzyne dimerization
Reaction Intermediate examples
Here are some of the examples of reaction intermediates:


|
Explore Courses for UPSC exam
|

|

















