Rules For Aromaticity | Chemistry Optional Notes for UPSC PDF Download
Four Key Rules For Aromaticity
There turn out to be 4 conditions a molecule must meet in order for it to be aromatic.
It’s all or nothing. If any of these conditions are violated, no aromaticity is possible.
- First, it must be cyclic.
- Second, every atom in the ring must be conjugated.
- Third, the molecule must have [4n+2] pi electrons (we’ll explain in depth what that means, below)
- Fourth, the molecule must be flat (usually true if conditions 1-3 are met, but there are rare exceptions)
Some example of aromatic compounds

Let’s go into more detail.
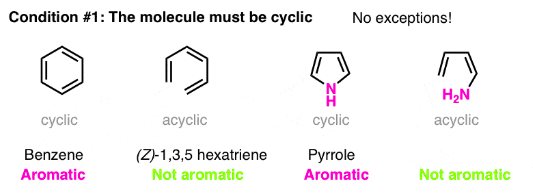
Condition #1 for Aromaticity: The Molecule Must Be Cyclic
Determining if a molecule is cyclic is pretty straightforward. Is there a ring? If yes, move to condition #2. If there’s no ring, forget it.
Case in point: (Z)-1,3,5 hexatriene has the same number of pi bonds (and pi electrons) as benzene, but isn’t aromatic. No ring, no aromaticity.
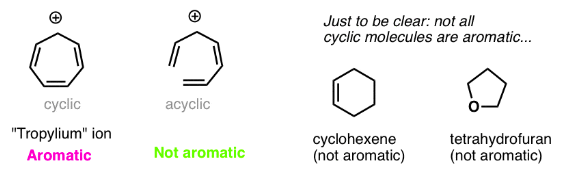
Condition #2 For Aromaticity: Every Atom In The Ring Must Be Conjugated
Obviously, being cyclic isn’t a sufficient condition for aromaticity. Just look at cyclohexene, above right. Not aromatic.
In order for aromaticty to exist, there must also be a continuous ring of p-orbitals around the ring that build up into a larger cyclic “pi system”.
One way of saying this is that every atom around the ring must be capable of conjugation with each other .
There are a few alternative ways to say the same thing.
We can also say:
- “Every atom in the ring must have an available p orbital”, or
- “Every atom in the ring must be able to participate in resonance”.
Remember that the “available p orbital” condition applies not just to atoms that are part of a pi bond, but also atoms bearing a lone pair, a radical, or an empty p orbital (e.g. carbocations).
The key thing that “kills” conjugation is an sp3 hybridized atom with four bonds to atoms. Such an atom cannot participate in resonance.
This is why the lone pair on pyrrole (below) isn’t as basic as you’d expect a nitrogen to be. Protonation of the nitrogen disrupts the conjugation around the ring, destroying aromaticity in the process.


Key thing to watch out for: an atom attached to four bonds e.g sp3 carbon or nitrogen
Condition #3 For Aromaticity: The Molecule Must Have [4n+2] Pi Electrons
The third condition is that the cyclic, conjugated molecule must have the correct number of pi electrons. Benzene and cyclooctatetraene are both cyclic and conjugated, but benzene is aromatic and cyclooctatetraene is not. The difference is that benzene has 6 pi electrons, and cyclooctatetraene has 8.

we often use is to say that benzene has [4n+2] pi electrons and cyclooctatetraene does not.
However, this term [4n+2] causes a lot of confusion in organic chemistry. I’ve known students who will stare at a molecule and try to figure out what “n” is.
“n” is not a property of the molecule!
Commenter Claire had a great way of putting it:
“4n+2 is not a formula that you apply to see if your molecule is aromatic. It is a formula that tells you what numbers are in the magic series. If your pi electron value matches any number in this series then you have the capacity for aromaticity.”
Exactly! The “magic series” is: 2, 6, 10, 14, 18, 22….. (and counting up from 4 after that).
[4n+2] is mathematical shorthand for writing out the series [2, 6, 10, 14, 18, 22…] .
We can generate this series by plugging in whole numbers (“n” = 0, 1, 2, 3, 4… ) to the [4n+2] formula. Those values of “n” have nothing to do with molecules. We are just using them to generate the series.
So for n = 0 , we have [4 (0) + 2] = 2
for n = 1 , we have [4 (1) + 2 ] = 6
for n = 2, we have [4 (2) + 2 ] = 10
for n = 3, we have [4 (3) +2 ] = 14
And so on. See how it generates the series [2, 6, 10, 14…] ? That’s the point of [4n+2].
The numbers in this “magic series” are sometimes referred to as “Hückel Numbers” after Erich Hückel, who proposed this rule back in 1931.
The condition that aromatic molecules must have [4n+2] pi electrons is sometimes called “Hückel’s rule”.
In the figure below, molecules which fulfill Hückel’s rule are in green; those which do not fulfill Hückel’s rule are in red.
- where [4n+2] is a formula describing the number series: 2, 6, 10, 14, 18, 22, 26....
- These are "magic numbers" for aromaticity: the number of pi electrons in the molecule must be in this series in order for the molecule to be aromatic
(We often call numbers in this series, "Huckel numbers”)

Another way of saying the same thing:
- the number of pi electrons is equal to an odd-numbered pair:
1 pair, 3 pairs, 5 pairs, 7 pairs.... (This also produces the series 2, 6, 10, 14 ...)
Note that we can count electrons in pi bonds as well as electrons from lone pairs (so long as the carbon isn’t already participating in a pi bond – see below). So the cyclopentadiene anion has six pi electrons – 4 from the two double bonds, and two from the lone pair on carbon.
Which Electrons Count As “Pi Electrons”, And Which Do Not?
That seems straightforward enough. However, complications can arise when we have atoms in the ring which both participate in pi bonding and also have a lone pair. For example,
- how do we count electrons in the benzene anion (below left) or pyridine? Do we count the lone pair electrons as Pi electrons, giving a total of 8? Or do we ignore them?
- What about furan (middle) which has two lone pairs on oxygen?
- What about pyrrole, with its lone pair on nitrogen, or imidazole, with two nitrogens?
How many Pi electrons are in these molecules?

In order to answer these questions, it’s important to remind ourselves of how p orbitals contribute to aromaticity in benzene.
In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Each p orbital contains a single electron. We can verify the total number of pi electrons in benzene by counting the pi bonds: 3 pi bonds times two electrons = 6 pi electrons total.
How p orbit Contribute To Aromaticity in Benzene

Note that the C-H bonds are at 90° to the pi system. If there was a lone pair where the C-H bond is, then it wouldn’t be able to interact with the pi system at all. Which brings us to….
Some Electrons Don’t Count: Pyridine and the Benzene Anion
The benzene anion has a lone pair on one of the carbons. This lone pair can’t be in a p orbital, since the p-orbital is participating in the pi system. Instead, it’s at 90 degrees to the pi system, in the plane of the ring.
In other words, the lone pair on carbon doesn’t count as a pair of pi electrons since it can’t overlap with the pi system.
The same is also true for pyridine, where the lone pair is also at right angles to the pi system.
So while in each case you might be tempted to say that they have 8 pi electrons, the correct answer is 6. This is a Hückel number, and both of these molecules are in fact aromatic.
Lone pairs in the "Benzene anion" and Pyridine are not actually "Pi electrons"
In the "benzene anion", the carbon with the lone pair is already contributing a p orbital (and one pi electron) to the pi system, just as in benzene. The lone pair is at 90° to the pi system (in the "plane of the ring") Since it can't overlap, its two electrons therefore don't count as "pi electrons"

The bottom line: each ring atom can contribute a maximum of one p orbital toward the pi system.
Some Examples of Aromatic 5-Membered Rings
Some molecules with five-membered rings can also present ambiguities.
The cyclopentadiene anion (below) has a lone pair on one of the carbons. Can this lone pair contribute to the pi system?
Since that carbon is not involved in any pi-bonding, the answer is yes.
The total number of pi electrons for the cyclopentadiene anion equals 2 (from the lone pair) plus the 4 electrons in the two pi bonds, giving us a total of 6. This is a Hückel number and the cyclopentadiene anion is in fact aromatic.
Cyclopentadiene anion: 6 pi electrons

- Here the lone pair can be part of the pi system Q since the carbon with the lone pair is not already contributing a p orbital to a pi bond.
- Lone pair contributes 2 electrons.
- When added to 4 pi electrons from the 2 pi bonds, we get 6 pi electrons total (a "Huckel number")
A similar situation arises for pyrrole. The nitrogen bears a lone pair but is not involved in a pi bond (unlike pyridine, above). Therefore it can contribute to the pi system and this gives us a total of 6 pi electrons once we account for the 4 electrons from the two pi bonds.
Pyrrole: 6 pi electrons

- Similar to cyclopentadiene anion.
- 2 electrons from lone pair contribute to pi system
- When added to 4 pi electrons from the 2 pi bonds,
we get 6 pi electrons total (a "Huckel number")
A curious case is furan, where the oxygen bears two lone pairs. Does this mean that furan has 8 pi electrons? No!
Why not? Because as we noted above, each atom can contribute a maximum of one p-orbital towards the pi system. In furan, one lone pair is in a p orbital, contributing to the pi system; the other is in the plane of the ring. This gives us a total of 6 pi electrons. Furan is aromatic. (So is thiophene, the sulfur analog of furan).
Furan: 6 pi electrons

Finally there is imidazole, which has two nitrogens. One nitrogen (the N-H) is not involved in a pi bond, and thus can contribute a full lone pair; the other is involved in a pi bond, and the lone pair is in the plane of the ring. This also gives us a total of 6 pi electrons once we account for the two pi bonds.

Condition #4 For Aromaticity: The Molecule Must Be Flat
The fourth condition for aromaticity is that the molecule must be flat (planar).
Aromaticity is such a stabilizing property (worth 20-36 kcal/mol) that generally a molecule that is
- cyclic
- conjugated
- has [4n+2] pi electrons
will also be flat. Give a molecule a large enough potential energy well, and it will fall into it eventually.
It’s a bit like the punch line to the old (crude) joke about why dogs adopt a certain energetically favourable conformation: “Because they can”.
As with certain vertebrates, the only thing that preventing a molecule that fulfills the first three conditions from being flat is if the flat conformation is incredibly strained. One example in this category is the molecule known as [10]-annulene, an isomer of which is drawn below left. In the trans, cis, trans, cis, cis isomer, the molecule is cyclic, conjugated, and has 10 pi electrons, but the two marked hydrogens bump into one another when attempting to adopt a flat conformation.
The molecule is prevented from adopting planarity due to this punitive Van Der Waals strain , and is therefore not aromatic.
Interestingly, if the hydrogens are removed and replaced with a bridging CH2 group, the strain is relieved and the pi bonds can adopt a planar conformation. The molecule below right shows the expected properties of an aromatic molecule.
- Generally, if the first three conditions are met then it's usually safe to assume that the molecule is flat. .
- A prominent exception is the isomer of [10]-annulene below left.

Although it is cyclic, conjugated, and has 10 pi electrons, it is not flat due to repuisions between hydrogens that arise when it is in the flat conformation.
Since it is not flat, it is not aromatic.

However, replacing the hydrogens with bonds to a bridging carbon allows all C-C pi bonds to be in the same plane - aromatic!
Summary: Rules For Aromaticity
This post went through the four conditions a molecule must meet to be aromatic.
Generally, determining if a molecule is cyclic and conjugated isn’t what trips people up.
It’s the damn pi electron-counting!
So in the next post, we’ll play the game show “Is This Aromatic?” with a series of different contestants and try to demonstrate a relatively quick n’ easy plan for determining if an unknown molecule is aromatic or not.
After that, we’ll look at the molecular orbitals and attempt to understand what exactly is so special about the Hückel series.
FAQs on Rules For Aromaticity - Chemistry Optional Notes for UPSC
| 1. What are the four key rules for aromaticity? |  |
| 2. Which atoms are considered as "pi electrons" in aromaticity? |  |
| 3. Can pyridine be considered aromatic? |  |
| 4. Can the benzene anion be considered aromatic? |  |
| 5. Can you provide some examples of aromatic 5-membered rings? |  |

|
Explore Courses for UPSC exam
|

|

















