Science Olympiad Model Test Paper - 2 | Olympiad Preparation for Class 10 PDF Download
Note: The questions provided in this document are similar to the questions that were asked in the actual Olympiad exam. So, we recommend you study these for your Olympiad preparation.
Logical Reasoning Section
Q1: Pointing to a boy on the stage, Aashi said, "he is the brother of the daughter of the wife of my husband". How is the boy on the stage related to Aashi?(a) Son
(b) Cousin
(c) Nephew
(d) Husband
 View Answer
View Answer 
Ans: (a)
Wife of Aashi’s husband - Aashi; Brother of daughter - Son.
So, the boy on the stage is Aashi’s son.
Q2: Pointing to a photograph, a woman said, his father is the only son of my daughter’s grandmother’s husband. How is the person in the photograph related to the woman?
(a) Husband
(b) Father
(c) Brother
(d) Son
 View Answer
View Answer 
Ans: (d)
Person’s father = Only son of woman’s daughter’s grandfather’s husband’s = Woman’s husband.
So, he is the son of the woman.
Q3: A word-and-number arrangement machine takes an input line of words and numbers and rearranges them according to specific rules in each step. The following is an example of input and the steps of rearrangement.
Input: my 84 very 46 efficient 32 mother 70
Step I: efficient my 84 very 46 32 mother 70
Step II: efficient 84 my very 46 32 mother 70
Step III: efficient 84 mother my very 46 32 70
Step IV: efficient 84 mother 70 my very 46 32
Step V: efficient 84 mother 70 my 46 very 32 And Step V is the final step of the above input. Based on the rules applied in the previous steps, answer the question. Step III of an input is "car 46 drives 28 slowly 32 he 16".
Which of the following is definitely the input?
(a) 46 car drives 32 he 16 28 slowly
(b) 46 car 32 drives he 16 28 slowly
(c) 46 car 32 drives 28 he 16 slowly
(d) Can't be determined
 View Answer
View Answer 
Ans: (d)
- The input line consists of words and numbers that are rearranged in a specific order.
- In Step III, the arrangement shows "efficient 84 mother my very 46 32 70".
- To determine the original input, we need to analyze the pattern of rearrangement.
- However, the exact original input cannot be definitively concluded from the given Step III.
- Thus, the answer is that it "Can't be determined".
Q4: P is the child of Q, and Q and R are siblings. T is R's mother. If S is T's son, which of the following statements is certainly true?
(a) T is the brother of Q.
(b) S is the cousin of P.
(c) Q and S are sisters.
(d) S is the maternal uncle of P.
 View Answer
View Answer 
Ans: (d)
- Understanding relationships: P is the son of Q, making Q his parent.
- Q and R are sisters, meaning they share the same parents.
- T is the mother of R, which makes T the mother of Q as well, since they are sisters.
- S is T's son, which means S is a brother to R and Q, making S the maternal uncle of P.
Q5: Complete the series;
2, 15, 4, 12, 6, 7, ?, ?
(a) 3, 8
(b) 8, 3
(c) 8, 0
(d) 8, 8
 View Answer
View Answer 
Ans: (c)
This sequence 2, 15, 4, 12, 6, 7, ?, ? is a combination of two series :
1st: 2, 4, 6, ? and 2nd: 15, 12, 7, ?
1st consists of consecutive even numbers.
So, missing term of 1st sequence = 8.
Pattern of 2nd sequence is -3, -5, ...
So, missing term of 2nd sequence = 7 - 7 = 0.
Q6: A girl was walking towards south and covered a distance of 7 m. She took a left turn and covered a distance of 10 m. Finally, she turned right and covered another 2 m. In which direction is she now from the starting point?
(a) South
(b) North-east
(c) South-west
(d) South-east
 View Answer
View Answer 
Ans: (d
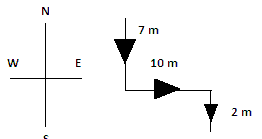 She is in the south-east direction from the starting point.
She is in the south-east direction from the starting point.
Q7: Two positions of a dice are given. Which number would be at the top when 2 is at the bottom?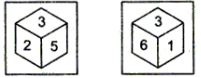 (a) 6
(a) 6
(b) 5
(c) 4
(d) 3
 View Answer
View Answer 
Ans: (a)
∴ Number 3 appears on the same face.
Hence, 2 is opposite to number 6 and 5 is opposite to number 1.
Q8: Directions: Complete the following analogy.
EFRT : GJXB : : DNSL : ?
(a) FRYT
(b) GRYT
(c) FSYT
(d) FRZT
 View Answer
View Answer 
Ans: (a)
The relation between EFRT and GJXB is as follows:-
E + 2 = G
F + 4 = J
R + 6 = X
T + 8 = B
In the same way, DNSL : FRYT
D + 2 = F
N + 4 = R
S + 6 = Y
L + 8 = T
Q9: In a specific coding system, the phrase 'birds are beautiful' is represented as 're pa si'; 'they fly in the sky' is represented as 'je ka ri so ta'; 'birds can fly' is represented as 'ka re mu', and 'sky is beautiful' is represented as 'so ve si'. Which of the following corresponds to the word 'beautiful'?
(a) je
(b) so
(c) si
(d) ve
 View Answer
View Answer 
Ans: (c)
- The word 'beautiful' appears in the phrases 'birds are beautiful' and 'sky is beautiful'.
- In the code, 'birds are beautiful' translates to 're pa si', and 'sky is beautiful' translates to 'so ve si'.
- From 'sky is beautiful', we see that 'si' is the common code for 'beautiful'.
- Thus, the correct code for 'beautiful' is 'si'.
Q10: Five friends P, Q, R, S, T take exams on different days (excluding Saturday and Sunday) of a week.
(i) R takes the exam the day right before S and right after T.
(ii) Q takes the exam in the 1st shift on the first day of the week.
(iii) P takes the exam in the 3rd shift.
(iv) S takes the exam in the 2nd shift on Friday.
(v) T takes the exam in the last shift.
Which of the following will take the exam on Tuesday?
(a) P
(b) Q
(c) R
(d) S
 View Answer
View Answer 
Ans: (a)
- From the clues, we know that Q takes the exam on Monday in the 1st shift.
- S takes the exam on Friday in the 2nd shift.
- Since R takes the exam just before S, R must take the exam on Thursday.
- That means T takes the exam on Wednesday in the last shift.
- Thus, P must take the exam on Tuesday in the 3rd shift.
Science Section
Q11: Two unequal masses P and Q are moving in straight lines and are stopped by applying the same retarding forces. If mass P takes twice the time of mass Q to stop, but only covers 41% of the distance that Q covers before stopping, what is the ratio of their initial velocities?(a) 1 : 2
(b) 1 : 8
(c) 2 : 3
(d) 2 : 1
 View Answer
View Answer 
Ans: (b)
- The problem states that mass P takes twice the time to stop compared to mass Q.
- However, P only covers 41% of the distance that Q does before stopping.
- Using the relationship between distance, time, and velocity, we can derive that the initial velocities of P and Q are in the ratio of 1:8.
- This means that for every 1 unit of velocity of P, Q has 8 units of velocity initially.
Q12: The essential difference between an AC generator and a DC generator is that
(a) AC generator has an electromagnet, while DC generator has permanent magnet.
(b) AC generator has slip rings, while DC generator has a commutator.
(c) AC generator gives higher voltage.
(d) DC generator gives higher voltage.
 View Answer
View Answer 
Ans: (b)
The only difference between an AC generator and a DC generator is that a split-ring commutator is used in DC generator, whereas two slip rings are used in AC generator.
Q13: A uniform electric field and a uniform magnetic field are produced, pointed in the same direction. An electron is projected with its velocity pointed in the same direction. Then,
(a) the electron will turn to its right
(b) the electron will turn to its left
(c) the electron velocity will decrease in magnitude
(d) the electron velocity will increase in magnitude
 View Answer
View Answer 
Ans: (c)
When the charge particle is moving parallel to the magnetic field, force exerted by the magnetic field on it will be zero. The force exerted by the electric field on the electron will be opposite to the direction of the electric field.
Hence, if the electron is moving parallel to the electric field and the magnetic field, the electron will move in the same straight direction with decreasing speed.
Q14: Read the given statements and select the correct option.
Statement 1: If the gravitational constant G continues to decrease over time, eventually the Earth will exit the solar system.
Statement 2: A decrease in G over time results in an increase in gravitational force.
(a) Both statements 1 and 2 are true, and statement 2 is the correct explanation of statement 1.
(b) Both statements 1 and 2 are true, but statement 2 is not the correct explanation of statement 1.
(c) Statement 1 is true, and statement 2 is false.
(d) Both statements 1 and 2 are false.
 View Answer
View Answer 
Ans: (c)
- Statement 1 is true because if the gravitational constant G were to decrease, the gravitational pull of the Earth would weaken, potentially allowing it to drift away from the solar system.
- Statement 2 is false because a decrease in G would actually mean that the gravitational force becomes weaker, not stronger.
- Thus, the correct option is that statement 1 is true while statement 2 is false.
Q15: Strips of metal X were dipped into two solutions as shown: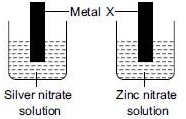
A greyish metallic deposit was found on both the strips. Which of the following could be metal X?
(a) Al
(b) Fe
(c) Cu
(d) Sn
 View Answer
View Answer 
Ans: (a)
Metals that are more reactive can displace the less reactive metals from their salt solution.
Reactivity series: K, Na, Ca, Mg, Al, Zn, Fe, Sn, Pb, [H], Cu, Hg, Ag, Au, Pt
The observation in the given activity that a grey deposit is seen in both the beakers indicates that metal 'X' is more reactive than both silver (Ag) and zinc (Zn) and can displace these metals from their salt solutions.
'X' is aluminium (Al).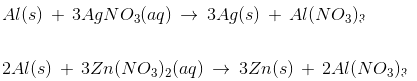
Q16: How much work is done in moving a charge of He2+ ions from a point at 118 volts to a point at 128 volts?
(a) 20 J
(b) 32 × 10-19 J
(c) 1.6 × 10-19 J
(d) 3.2 × 10-19 J
 View Answer
View Answer 
Ans: (b)
We know, potential difference, V = W/Q
Here, potential difference, V = 128 – 118 = 10 volts
Work done, W = ?
Charge on the He2+ ion = 2 × 1.6 × 10-19 = 3.2 × 10-19 C
Hence, work done, W = VQ = 10 × 3.2 × 10-19 = 32 × 10-19 J
Q17: In a certain population of rabbits, there are two alleles for coat colour, brown and white. Brown is dominant and white is recessive. Wolves are also present in the area and rabbits constitute a major portion of their diet. If the climate were to change such that snow covered the ground for much of the time, what change would you expect from the options given below?
(a) The frequency of white allele would increase.
(b) The frequency of brown allele would increase.
(c) The frequency of white allele would decrease.
(d) None of the above
 View Answer
View Answer 
Ans: (a)
In order to survive, there will be genetic variation in the allele frequency. As an adaptive response for camouflage, the frequency of white allele will increase. The rabbits with white fur will be better camouflaged than the rabbits with brown fur. Hence, the frequency of white allele will increase.
Q18: Read the given statements and select the correct option.
Statement 1: The loudness or softness of a sound is determined basically by its frequency.
Statement 2: The amplitude of the sound wave depends upon the intensity with which an object is made to vibrate.
(a) Both statements 1 and 2 are true, and statement 2 is the correct explanation of statement 1.
(b) Both statements 1 and 2 are true, but statement 2 is not the correct explanation of statement 1.
(c) Statement 1 is false, but statement 2 is true.
(d) Both statements 1 and 2 are false.
 View Answer
View Answer 
Ans: (c)
- Statement 1 is incorrect because loudness is actually determined by the amplitude of the sound wave, not its frequency.
- Statement 2 is correct as it accurately describes that the amplitude of a sound wave is related to how intensely an object vibrates.
- Thus, the correct option is that Statement 1 is false and Statement 2 is true.
Q19: An elongated object is placed in front of a concave mirror between its pole and focus. As the object moves towards the pole,
(I) Speed of the image is more than the speed of the object.
(II) The image becomes enlarged.
(III) The image approaches the mirror.
(IV) The image becomes diminished.Choose the correct statements:
(a) I and II
(b) I and III
(c) I, II and III
(d) I, III and IV
 View Answer
View Answer 
Ans: (d)
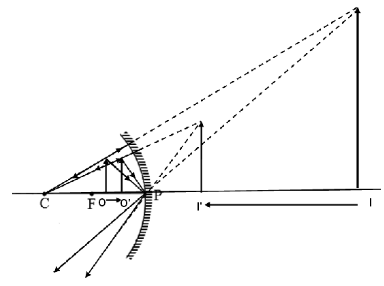
From the figure we can conclude that the image formed is erect and virtual. As the object moves from O to O', the image approaches the mirror from I to I'.
Size of the image becomes less and the distance travelled by the image is more than the distance travelled by the object in the same time, so the speed of the image is more than the speed of the object.
Q20: A line with a total resistance of 0.5Ω supplies 20kW at 220V to a small industrial facility. What is the efficiency of the transmission?
(a) 79.6%
(b) 76.1%
(c) 82.9%
(d) 92.2%
 View Answer
View Answer 
Ans: (c)
- To find the efficiency, we first calculate the input power using the formula: Input Power = Output Power + Power Loss.
- The output power is given as 20kW. The power loss can be calculated using the formula: Power Loss = I²R, where R is the resistance (0.5Ω).
- First, we find the current (I) using the formula: P = VI, where P is the output power (20,000W) and V is the voltage (220V). This gives us I = 20,000W / 220V = 90.91A.
- Now, calculate the power loss: Power Loss = (90.91A)² x 0.5Ω = 4131.5W or approximately 4.13kW.
- Now, Input Power = 20kW + 4.13kW = 24.13kW. Finally, efficiency = (Output Power / Input Power) * 100 = (20kW / 24.13kW) x 100 = 82.9%.
Q21: Which of the following is an example of a hazardous waste?
(a) Iron nails
(b) Metal scraps
(c) Petroleum
(d) Chlorine bleach
 View Answer
View Answer 
Ans: (d)
- Hazardous waste is waste that poses a substantial and potential threat to public health or the environment. In the United States, the treatment, storage, and disposal of hazardous waste are regulated under the Resource Conservation and Recovery Act (RCRA). The most common examples of hazardous waste found within the homes include paints, batteries, solvents, cleaning agents and pesticides.
- Among the listed substance, chlorine bleach is a chemical which is hazardrous, if not disposed properly.
Q22: The transmission of a nerve impulse across a synapse occurs by
(a) covalent bond formation between two dendrites
(b) diffusion of neurotransmitter across the cleft
(c) physical contact of axon to dendrite
(d) movement of potassium ions across the cleft
 View Answer
View Answer 
Ans: (b)
Synaptic transmission relies on the availability of the neurotransmitter; the release of the neurotransmitter by exocytosis; the binding of the postsynaptic receptor by the neurotransmitter; the functional response of the postsynaptic cell; and the subsequent removal or deactivation of the neurotransmitter.
Q23: A metallic wire is connected in series with a DC battery source of +12 V. If the radius and length of the wire are doubled, what effect will it have on the resistance of the wire and current through the wire?
(a) The resistance and current will be halved.
(b) The resistance will be doubled and the current will be halved.
(c) The resistance will be halved and the current will be doubled.
(d) The resistance will be four times and the current will be halved.
 View Answer
View Answer 
Ans: (c)
The resistance of a wire is given by:
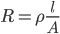
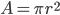
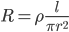
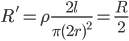
The battery voltage will remain the same.
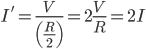
Thus, the current will be doubled.
Q24: If 10 g of each of the following substances is taken, then which one of them will contain the least number of atoms? (Atomic mass of C = 12 u, Na = 23 u, H = 1 u, F = 19 u)
(a) Methane
(b) Sodium
(c) Hydrogen fluoride
(d) Carbon
 View Answer
View Answer 
Ans: (b)
- To find out which substance has the least number of atoms, we need to calculate the number of moles for each substance using the formula: moles = mass/atomic mass.
- For Sodium (Na): 10 g / 23 u = 0.434 moles, which equals approximately 0.434 x 6.022 x 1023 atoms = 2.61 x 1023 atoms.
- For Methane (CH4): 10 g / (12 + 4x1) = 10 g / 16 u = 0.625 moles, which equals approximately 0.625 x 6.022 x 1023 atoms = 3.76 x 10^23 atoms.
- For Hydrogen fluoride (HF): 10 g / (1 + 19) = 10 g / 20 u = 0.5 moles, which equals approximately 0.5 x 6.022 x 1023 atoms = 3.01 x 1023 atoms.
- For Carbon (C): 10 g / 12 u = 0.833 moles, which equals approximately 0.833 x 6.022 x 1023 atoms = 5.01 x 1023 atoms.
- Among these calculations, Sodium has the least number of atoms at approximately 2.61 x 1023 atoms.
Q25: In which of the following parts of the plants is apical meristem found?
(a) Root apex
(b) Side of the stem
(c) Base of leaf
(d) Side of the node
 View Answer
View Answer 
Ans: (a)
Apical meristem is present on root apex, stem apex, leaf buds and flower buds. They are responsible for growth in length, i.e. primary growth.
Q26: Phagocytosis is the ingestion of bacteria or other material by phagocytes and amoeboid protozoans. High abundance of which of the following organelles in their cytosol will help in the process?
(a) Golgi bodies
(b) Endoplasmic reticulum
(c) Mitochondria
(d) Centriole
 View Answer
View Answer 
Ans: (c)
Phagocytosis is an energy requiring process. The mitochondria is responsible for the production of ATP. Hence, high number of mitochondria gives enough energy for the process.
Q27: Which of the following displays the wrong sequence of the specified property?
(a) Atomic radii - F < O < C < Cl < Br
(b) Metallic character - Na < Mg < Al < Si
(c) Acidic nature - Al₂O₃
(d) Non-metallic character - F > Cl > Br > I
 View Answer
View Answer 
Ans: (b)
- In option (b), the metallic character is listed as Na < Mg < Al < Si, which is incorrect because aluminum (Al) is more metallic than silicon (Si).
- In contrast, the other options correctly represent the trends in atomic radii, acidic nature, and non-metallic character.
- Thus, option (b) is the only one that does not follow the correct order.
- The question asks for the incorrect order of a property.
Q28: Select the combination of isobars and isotopes from the following species: 1737 P, 1836 Q, 1735 R, 1937 S, 1838 T
(a) P, S and Q, R
(b) P, Q
(c) P, S
(d) P, S
 View Answer
View Answer 
Ans: (c)
- Isobars are atoms with the same mass number but different atomic numbers. In this case, P (1737) and S (1937) are isobars.
- Isotopes are atoms of the same element with different mass numbers. Here, P (1737) and Q (1836) are isotopes.
- Thus, the correct pair of isobars and isotopes is P and S.
- Option (c) correctly identifies this pair.
Q29: Which of the following statements is accurate?
(a) When CaSO₄ is mixed with water, it produces a solution with pH greater than 7.
(b) The aqueous solution of K₂CO3 has a pH equal to 7.
(c) The aqueous solution of CH₃COOK will have a pH greater than 7.
(d) The aqueous solution of CH₃COONa will have a pH less than 7.
 View Answer
View Answer 
Ans: (c)
- The correct answer is (c) because CH₃COOK (potassium acetate) is a salt derived from a weak acid (acetic acid) and a strong base (potassium hydroxide). This results in a solution that is basic, hence the pH is greater than 7.
- In contrast, CaSO₄ does not significantly affect the pH when dissolved, and K₂CO3 is a basic salt, so its pH is also greater than 7, not equal to 7.
- Lastly, CH₃COONa (sodium acetate) also results in a basic solution, not an acidic one.
Q30: A lens is used as a simple magnifier having magnification 5. Find the focal length of the lens.
(a) +5
(b) -5
(c) +25
(d) -25
 View Answer
View Answer 
Ans: (a)
A convex lens can be used as a simple magnifier if the object is placed between focal and optical centre.
Magnification of the magnifier, m = D/f; where D is the least distance of distinct vision.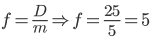
Q31: Junction between two neurons is called
(a) cell junction
(b) neuro muscular junction
(c) neural joint
(d) synapse
 View Answer
View Answer 
Ans: (d)
Synapse is the junction between two neurons. In the nervous system, a synapse is a structure that permits a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron.
Q32: What is the number of atoms of Mg, S, and O in 0.25 moles of MgSO₄? (Given: Atomic mass of O = 16 u, Mg = 24 u, S = 32 u)
(a) 1.505×10²³, 1.505×10²³ and 6.023×10²³
(b) 6.023×10²³, 3.01×10²³ and 1.2×10²³
(c) 3.01×10²³, 1.05×10²³ and 12.04×10²³
(d) 1.505×10²³, 12.05×10²³ and 12.04×10²³
 View Answer
View Answer 
Ans: (a)
- To find the number of atoms in 0.25 moles of MgSO₄, we first note that each formula unit contains 1 Mg, 1 S, and 4 O atoms.
- Calculating the total atoms: For Mg, it is 0.25 moles × 6.022×10²³ = 1.505×10²³ atoms.
- For S, it is also 0.25 moles × 6.022×10²³ = 1.505×10²³ atoms.
- For O, it is 0.25 moles × 4 × 6.022×10²³ = 6.023×10²³ atoms.
- Thus, the number of atoms of Mg, S, and O are 1.505×10²³, 1.505×10²³, and 6.023×10²³, respectively.
Q33: In some organisms, the unfertilised egg can develop into a new individual. What is this development without fertilisation called?
(a) Totipotency
(b) Emasculation
(c) Parthenogenesis
(d) Hybridisation
 View Answer
View Answer 
Ans: (c)
In some organisms, the unfertilised egg can develop into a new individual. This type of development without fertilisation is called parthenogenesis. Examples: Rotifers, drones of honeybees, etc.
Q34: Consider the following diagram:
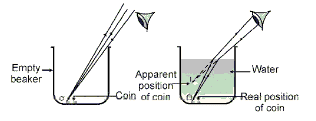
In the given diagram, why does the coin placed at the bottom of the beaker containing water appear to be raised. Choose the correct reason from the following options.
(a) This is due to the refraction of light.
(b) A ray is travelling from optically less denser medium to more denser medium.
(c) The ratio of perpendicular in air to the ratio of perpendicular in the denser medium is constant.
(d) The refracted ray is travelling from denser medium to rarer medium.
 View Answer
View Answer 
Ans: (a)
It is due to refraction of the light. Refraction is the phenomenon in which there is a change in the path of the light with change in the medium.
In the given case, the ray of the light travels from denser medium to rarer medium. It bends away from the normal, so the coin will appear to be raised.
Q35: Which of the following is the chromosomal attachment of the spindle microtube?
(a) Telomere
(b) Centromere
(c) Centrosome
(d) Liposome
 View Answer
View Answer 
Ans: (b)
Centromere is the region of a chromosome to which the microtubules of the spindle attach, via the kinetochore, during cell division. During prophase, the spindle also begins to form as the two pairs of centrioles move to opposite poles and microtubules begin to polymerise from the duplicated centrosome.
Q36: According to Mendel's Law of Dominance, what does it state about contrasting characters?
(a) Out of two contrasting characters, only one expresses itself in F₁ generation
(b) Out of two contrasting characters, one is eliminated while the other is shown in F₁ generation
(c) Inherited factors controlling a specific trait are interdependent
(d) Both dominant and recessive phenotypic traits are shown in F₁ generation
 View Answer
View Answer 
Ans: (a)
- Mendel's Law of Dominance explains that when two different traits are crossed, only one trait will be visible in the first generation (F₁).
- This means that the dominant trait overshadows the recessive one, making it appear as if the recessive trait is not present.
- In simple terms, if you have a tall plant and a short plant, the offspring will only show the tall trait.
- This principle is fundamental in understanding how traits are inherited in genetics.
Q37: Which of the following traits examined by Mendel is considered recessive?
(a) Green color of seed
(b) Tall plant size
(c) Violet flower color
(d) Wrinkled pod shape
 View Answer
View Answer 
Ans: (a)
- Green color of seed is a trait that Mendel found to be recessive, meaning it only appears when both alleles are for green seeds.
- The other traits, such as tall plant size, violet flower color, and inflated pod shape, are dominant traits.
- This means that if a plant has at least one dominant allele for these traits, they will be expressed.
- In contrast, the recessive trait will only show up if the plant has two recessive alleles.
Q38: Study the tables related to blood sugar levels.
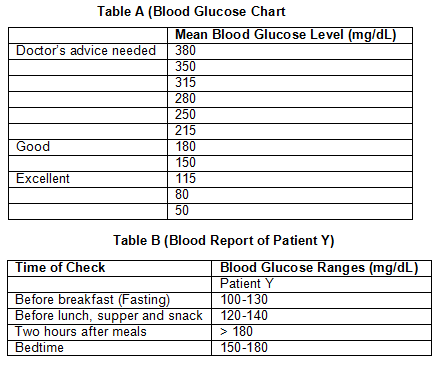
Malfunctioning of which organ is responsible for the disease that can be inferred by the data given above?
(a) Ovary/Testis
(b) Kidney
(c) Liver
(d) Pancreas
 View Answer
View Answer 
Ans: (d)
The disease that can be inferred from the given data is diabetes.
Diabetes is caused due to insufficient production of insulin by the beta cells of the pancreas.
Q39: Given below is a list of several negative impacts on humans.
(i) Cancer
(ii) Cataract in eyes
(iii) Decreased body's ability to fight diseases
(iv) Higher mortality rate of developing embryos.
Which of these are linked to ozone depletion?
(a) (i) and (iii) only
(b) (ii) and (iv) only
(c) (iii) and (iv) only
(d) (i), (ii), (iii), and (iv)
 View Answer
View Answer 
Ans: (d)
- The ozone layer protects us from harmful ultraviolet (UV) radiation from the sun.
- When the ozone layer is depleted, it can lead to serious health issues such as cancer and cataracts.
- Additionally, it can weaken the body's immune system, making it harder to fight off diseases.
- It can also affect developing embryos, leading to increased mortality rates.
- Thus, all the listed effects (i, ii, iii, and iv) are caused by ozone depletion.
Q40: It is not advised to store curd and other sour substances in copper or brass vessels because
(a) curd and sour substances contain acids
(b) curd and sour substances contain bases which are bitter
(c) curd and sour substances lose their flavour and freshness if preserved in metal containers
(d) curd and sour substances rot if preserved in metal containers
 View Answer
View Answer 
Ans: (a)
All sour substances like curds and pickles contain acids, and acids react with metals.
Such food if kept in brass or copper vessels will cause chemical reactions, which are likely to yield toxic products
Q41: Select the accurate pairing.
(a) Bhakra Dam - Satluj river
(b) Tehri Dam - Tawa river
(c) Sardar Sarovar Dam - Kaveri river
(d) Mettur Dam - Narmada river
 View Answer
View Answer 
Ans: (a)
- The Bhakra Dam is located on the Satluj river, making this pairing correct.
- The Tehri Dam is actually on the Bhagirathi river, not Tawa.
- The Sardar Sarovar Dam is built on the Narmada river, not Kaveri.
- The Mettur Dam is situated on the Kaveri river, not Narmada.
Q42: The role of the seminal vesicle is to:
(a) secrete follicle-stimulating hormone
(b) facilitate sperm movement within the female reproductive system
(c) supply energy to sperm
(d) secrete testosterone
 View Answer
View Answer 
Ans: (c)
- The seminal vesicle plays a crucial role in the male reproductive system.
- It primarily functions to provide energy to sperm, which is essential for their motility.
- Additionally, it helps in making the transport of sperm easier within the female reproductive tract.
- However, it does not secrete follicle-stimulating hormone, which is produced by the pituitary gland.
Q43: The three pairs of salivary glands that secrete into the oral cavity are
(a) alpha, beta, and gamma
(b) parotid, submandibular, and sublingual
(c) palatine, lingual, and pharyngeal
(d) serous, mucous, and mixed
 View Answer
View Answer 
Ans: (b)
The salivary glands in mammals are exocrine glands, glands with ducts, that produce saliva. The 3 types of salivary glands are parotid, submandibular and sublingual glands.
Q44: Given below is a table of differences between rough endoplasmic reticulum and smooth endoplasmic reticulum. RER
(i) It consists mainly of tubules and vesicles.
(ii) It has ribosomes attached on its cytoplasmic surface.
(iii) It is well developed in cells specialized to synthesize proteins.
(iv) The products do not pass into the lumen of the endoplasmic reticulum. Select the incorrect pair of differences.
(a) (i) and (ii) only
(b) (ii) and (iv) only
(c) (i) and (iv) only
(d) (ii) and (iii) only
 View Answer
View Answer 
Ans: (c)
- The rough endoplasmic reticulum (RER) is characterized by the presence of ribosomes on its surface, which is why it is termed "rough".
- Statement (i) is correct as RER does consist of tubules and vesicles.
- Statement (iv) is incorrect because the products synthesized in the RER do indeed pass into the lumen for further processing.
- Thus, the incorrect pair of differences is (i) and (iv), making option (c) the right choice.
Q45: Which of the following is an example of non-point source water pollution?
(a) Factories
(b) Runoff water from fields, roads, etc.
(c) Oil wells
(d) Wastewater treatment plants
 View Answer
View Answer 
Ans: (b)
- The correct answer is runoff water from fields, roads, etc. because it comes from multiple sources and is not easily traceable to a single point.
- This type of pollution occurs when rainwater or melting snow washes pollutants off surfaces and into water bodies.
- In contrast, factories and oil wells are considered point sources because they discharge pollutants from a specific location.
- Understanding the difference between point and non-point sources is crucial for effective water pollution management.
Achievers Section
Q46: Read the given paragraph where a few words are italicized and select the incorrect statement regarding them.The most vital function of the heart is heartbeats. Sequence of events which takes place during the completion of one heartbeat is called cardiac output. It involves rhythmic contraction and relaxation of heart muscles. Contraction is called diastole and relaxation is called systole. Atrial contraction forces pumping of oxygenated blood from the right atrium to the right ventricles. During contraction of ventricles, blood from the right ventricles flows to the lungs through the aorta and blood from the left ventricle is distributed to all parts of the body through the pulmonary artery.
(a) Cardiac output must be changed to cardiac cycle.
(b) Diastole and systole must be interchanged.
(c) Oxygenated must be changed to deoxygenated.
(d) Aorta and pulmonary artery must not be changed as they are correctly mentioned.
 View Answer
View Answer 
Ans: (d)
- The heart's function is crucial, and the paragraph explains the heartbeat process.
- The term cardiac output is correctly used to describe the events of a heartbeat.
- Diastole refers to the heart's relaxation, while systole refers to contraction; they are correctly defined.
- The aorta and pulmonary artery are correctly identified in their roles in blood circulation.
Q47: A rod is placed before a concave mirror having radius of curvature 20 cm as shown in the figure below. Find the total length of the image.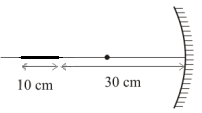 (a) 5 cm
(a) 5 cm
(b) 3.4 cm
(c) 1.7 cm
(d) 1.3 cm
 View Answer
View Answer 
Ans: (c)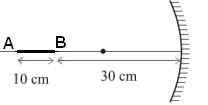 Considering the image of the point A,
Considering the image of the point A,
u = -40 cm, f = -10 cm
Using mirror formula: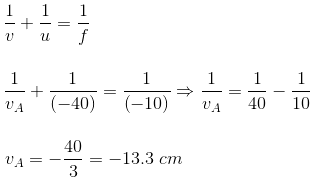
Considering the image of the point B,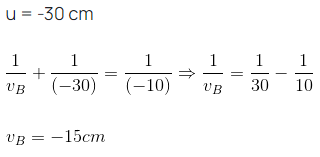
Length of the image = vB - vA = 15 - 13.3 = 1.7 cm
Q48: Analyse the two statements given below and select the correct option.
Statement I: Soaps are sodium salts of higher fatty acids.
Statement II: Sodium chloride is one of the chief materials used for the preparation of soap.
(a) Both statements I and II are correct, but statement II is not the correct reason for I.
(b) Both statements I and II are correct and statement II is the correct reason for I.
(c) Statement I is correct, but statement II is incorrect.
(d) Both statements I and II are incorrect.
 View Answer
View Answer 
Ans: (a)
Soaps are sodium or potassium salts of higher fatty acids. They are prepared by heating vegetable oils (esters of higher fatty acids) with alkalies (NaOH or KOH).
Vegetable oils + NaOH → Soap + Glycerol
This reaction is called saponification.
Once soap is formed, NaCl is added to help in precipitation of the soap.
Hence, both statements I and II are correct, but statement II is not the correct reason for I.
Q49: According to the given image below, which of the following is/are precaution(s) that is/are true to be considered in order to determine the focal length of a concave mirror by obtaining the image of a distant object?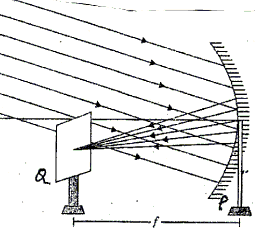
(i) The base of the concave mirror stand and the screen should not be parallel to the metre scale.
(ii) The concave mirror should be fixed in the vertical plane.
(iii) Place the screen behind the mirror.
(a) Only (i)
(b) Only (ii)
(c) Both (i) and (iii)
(d) Only (iii)
 View Answer
View Answer 
Ans: (b)
To determine the focal length of a concave mirror by obtaining the image of distant object, place the screen in front of the mirror and the concave mirror should be fixed in the vertical plane.
The base of the concave mirror stand and the screen should be parallel to the metre scale.
Q50: Which of the following statements is/are incorrect?
I. Out of T and U, U is bigger in size as it has more number of electrons.
II. Metal T has valency 3 as it belongs to group 13.
III. R and W are called noble gases.
(a) I and III only
(b) I only
(c) II and III only
(d) I, II, and III
 View Answer
View Answer 
Ans: (b)
- Statement I is incorrect because size is not solely determined by the number of electrons; it also depends on the number of protons and the overall atomic structure.
- Statement II is correct as metals in group 13 typically have a valency of 3.
- Statement III is incorrect because R and W are not noble gases; noble gases are elements like helium, neon, and argon.
- Thus, the only incorrect statement is I, making option (b) the correct answer.
|
70 videos|242 docs|187 tests
|
FAQs on Science Olympiad Model Test Paper - 2 - Olympiad Preparation for Class 10
| 1. What topics are covered in the Science Olympiad Model Test Paper - 2 for Class 10? |  |
| 2. How can I prepare effectively for the Science Olympiad? |  |
| 3. What is the format of the Science Olympiad exam? |  |
| 4. Are there any specific strategies for solving logical reasoning questions in the Olympiad? |  |
| 5. How important is time management during the Science Olympiad exam? |  |




















