Short & Long Answer Questions: Surface Chemistry - 2 | Physical Chemistry for NEET PDF Download
Q.51 Give one example each of oil in water and water in oil type emulsions.
Answer: Milk and vanishing cream (o/w type) cream and butter (w/o type).
Q.52 What is emulsifying agent? Give one example.
Answer: The substance which stabilizes the emulsion of oil in water or water in oil is called emulsifying agent e.g. proteins, gums, alcohols etc.
Q.53 Why does sky look blue?
Answer: Dust particles along with water suspended in air scatter blue light which reaches our eyes and the sky looks blue to us.
Q.54 Give reason for the formation of delta.
Answer: River water is a colloidal solution of clay. Sea water contains a number of electrolytes. When river water meets the sea water, the electrolytes present in water of sea coagulate the colloidal solution of clay resulting in its deposition with the formation of delta.
Q.55 How is natural rubber obtained?
Answer: Natural rubber is obtained by coagulation of latex by acetic acid.
Q.56 What is the use of emulsion in photography?
Answer: Photographic plates or films are prepared by coating an emulsion of the light sensitive silver bromide in gelatin over glass plates or celluloid films.
Q.57 What is tanning?
Answer: Animal hides are colloidal in nature. When a hide which has positively charged particles is soaked in tannin, which contains negatively charged colloidal particles, mutual coagulation takes place. This results in the hardening of leather. This process is termed as tanning.
Q.58 Colloidal medicines are more effective. Why ?
Answer: Colloidal medicines are more effective because they have large surface area and therefore easily assimilated.
Q.59 What happens when alum is added to natural water?
Answer: When alum is added to natural water it coagulates the suspended impurities and make water fit for drinking purpose.
Q.60 When does rainfall occur?
Answer: Clouds are aerosols having small droplets of water suspended in air. On account of condensation these droplets become bigger in size and fall down. Sometimes rain fall occurs when two oppositely charged clouds meet.
Q.61 What is difference between a 'sol' and a 'gel'?
Answer: Those colloids in which dispersed phase is solid and dispersion medium is liquid is called sol. Those colloids in which dispersed phase is liquid and dispersion medium is solid is called gel.
Q.62 What is adsorption?
Answer: The accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed as adsorption.
Q.63 What is absorption? How is absorption different from adsorption?
Answer: The process in which a liquid or gas is uniformly distributed into whole of another substance is called absorption. The accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed as adsorption. In adsorption, the adsorbate is concentrated only at the surface and doesn't penetrate through the surface to the bulk of the adsorbent. While in absorption the substance is uniformly distributed throughout the bulk of the solid
Q.64 What are the points of difference between physisorption and chemisorption?
Answer: Points of difference between physisorption and chemisorptions :
| Physisorption | Chemisorption |
| It arises due to van der Wassls forces. | It is caused by chemical bond formation. |
| It is not specific in nature. | It is highly specific in nature. |
| It is reversible in nature. | It is irreversible. |
| Low temperature is favourable. It decreases with increasing temperature. | High temperature is favorable. It increases with increasing temperature. |
Q.65 On what factors adsorption of a solute from a solution varies?
Answer: Following factors affect adsorption of solute from solution
(i) The extent of adsorption decreases with an increase in temperature.
(ii) It increases with an increase of surface area of the adsorbent.
(iii) It also depends on the increase in the concentration of the solute in the solution.
(iv)It also depends on nature of adsorbent and adsorbate.
Q.66 Define Catalysis.
Answer: The process of mixing foreign chemical substance which alters the rate of a chemical reaction and itself remains chemically and quantitatively unchanged after reaction is called catalysis.
Q.67 What is the mechanism of heterogeneous catalysis?
Answer: The mechanism of heterogeneous catalysis involves five steps :
(i) Diffusion of reactant to the surface of catalyst.
(ii) Adsorption of reactant molecules on the surface of the catalyst.
(iii) Occurrence of chemical reaction on the catalyst surface through formation of an intermediate.
(iv) Desorption of products from the catalyst surface and thereby the surface available again for more reaction to occur.
(v) Diffusion of reaction products away from catalyst's surface.
Q.68 What is intermediate compound formation theory of catalysis?
Answer: According to this theory of homogeneous catalysis, the catalyst combines with one of the reactants and forms unstable intermediate. This intermediate reacts with another reagent or decomposes to give products.
Q.69 What is shape selective catalysis?
Answer: The catalytic reaction that depends upon the pore structure and size of the catalyst and the size of the reactant and product molecule is called shape-selective catalysis.
Q.70 What is the mechanism of enzyme catalysis?
Answer: The molecules of reactants, which have complementary shape, fit into the cavities of enzymes just like a key fits into a lock. On account of the presence of active groups, an activated complex is formed which then decomposes to yield the products.
Q.71 How are colloids classified on the basis of physical state of dispersed phase and dispersion medium?
Answer: On the basis of physical state of dispersed phase and dispersion medium colloids are of 8 types
| Dispersed | Dispersion medium | Type of Colloid |
| Solid Solid Solid Liquid Liquid Liquid Gas Gas | Solid Liquid Gas Solid Liquid Gas Solid Liquid | Solid sol Sol Aerosol Gel Emulsion Aerosol Solid sol Foam |
Q.72 What are lyophilic and lyophobic colloids? Give one example of each type.
Answer: A colloidal sol in which dispersed phase and dispersion medium attract each other is called lyophilic colloid, e.g. gum. A colloidal sol in which dispersed phase and dispersion medium repel each other is called lyophobic colloid, e.g. gold solution.
Q.73 What are micelles? Give one example of a micelle system.
Answer: Some substances which at low concentration behave as normal strong electrolytes but at high concentration exhibit colloidal behaviour due to the formation of aggregates. These aggregates are called micelles, e.g. soaps.
Q.74 What is peptization?
Answer: The process of converting a precipitate into colloidal solution by shaking it with dispersion medium in the presence of small amount of electrolyte is called peptization.
Q.75 What is dialysis? Mention its use.
Answer: Dialysis is the process of removing a dissolved substance from a colloidal solution by means of diffusion through a suitable membrane. It is used in purification of blood in kidney.
Q.76 Explain ultra filtration.
Answer: ultra filtration is the process of separating the colloidal solution from soluble solute present in the colloidal solution by specially prepared filters, which are permeable to all substances except the colloidal particles.
Q.77 What is Tyndall effect?
Answer: The scattering of light by colloidal particles is termed as Tyndall effect.
Q.78 What are the two conditions necessary for Tyndall effect?
Answer: Two conditions necessary for Tyndall effect are (i) Wavelength of light should be in the range of diameter of colloidal particles. (ii) Refractive indices of the dispersed phase and dispersion medium should differ greatly in magnitude.
Q.79 What do you mean by Brownian movement?
Answer: The zig-zag motion of colloidal sol particles in all possible directions is known as Brownian movement.
Q.80 What is the cause of Brownian movement?
Answer: The Brownian movement is due to unbalanced bombardment of the solution particles by the molecules of the dispersion medium.
Q.81 What is the reason for origin of charge on colloidal particles?
Answer: The reasons for origin of charge on colloidal particles (i) Electron capture by solution particles during electro dispersion. (ii) Selective adsorption of ion by the sol from solution. (iii) Frictional electrification
Q.82 Explain electrophoresis.
Answer: Migration of colloidal sol particles towards oppositely charged electrodes under electric field is called electrophoresis.
Q.83 What is electro-osmosis?
Answer: Movement of dispersion medium towards an electrode under electric field through semi permeable membranes is called electro osmosis.
Q.84 Define coagulation.
Answer: The process of setting of colloidal solution particles is known as coagulation.
Q.85 State Hardy Schulze rule.
Answer: Coagulation power of an ion to coagulate colloidal solution of opposite charge is directly proportional to fourth power of its valency.
Q.86 Write three important differences between lyophilic a lyophobic colloids.
Answer: Difference between lyophilic and lyophobic colloids
| Lyophobic colloid | Lyophilic colloid | |
| 1 | These are solvent repelling | These are solvent attracting. |
| 2 | These are irreversible. | These are reversible. |
| 3 | These are unstable. | These are stable. |
Q.87 Explain with example (i) Multimolecular colloids (ii) Macromolecular colloids and (iii) Associated colloids.
Answer: (i) Multimolecular colloids : A colloid in which large number of small molecules combine to form a particle of colloidal size is called multimolecular colloid, e.g. gold sol. (ii) Macromolecular colloids : A colloid in which size of the molecule is in colloidal range is called macromolecular colloid, e.g. starch, protein (iii) Associated colloids : There are some substances which at low concentration behave as true solution and at higher concentration exhibit colloidal behavior are called associated colloids. e.g. soap.
Q.88 Pressure of a closed vessel filled with gas decreases when powdered charcoal is added.
Answer: Powdered charcoal is a good adsorbent. It adsorbs the gases on its surface and reduces the pressure of gas in the vessel,
Q.89 Explain the cause of adsorption.
Answer: Adsorption arises due to the fact that the surface particles of the adsorbent are not in the same environment as the particles inside the bulk. In side the adsorbent all the forces acting between the particles are mutually balanced but on the surface the particles are not surrounded by atoms or molecules of their kind on all sides and hence they possess unbalanced or residual attractive forces. These forces are responsible for attracting the adsorb ate particles on its surface.
Q.90 Why is necessary to purify the colloidal solution?
Answer: The presence of traces of electrolyte is essential for the stability of the colloidal solution but larger quantities coagulate it so, it is necessary to purify it.
Q.91 Physical and chemical adsorption respond differently to the rise in temperature. Explain.
Answer: Both physical and chemical adsorption are of exothermic nature. The extent of adsorption is likely to decrease with rise in temperature. However, in chemical adsorption, the bonds present in the reactant species are to cleave initially and this is followed by adsorption. Since bond cleavage requires some energy, the chemical adsorption initially increases with rise in temperature and then decreases. Physical adsorption however, decreases with rise in temperature. For details consult Section 5.5.
Q.92 Identify the dispersed phase and dispersion medium in the following colloidal solutions : (i) smoke (ii) fog (iii) cheese (iv) milk (v) whipped cream.
Answer: (i) Smoke: solid particles dispersed in air (solid/gas)
(ii) Fog : Water drops dispersed in air (liquid/gas)
(iii) Cheese : Liquid dispersed in solid fat (liquid/solid)
(iv) Milk : Liquid dispersed in liquid (emulsion)
(v) Whipped cream : Air dispersed in liquid (gas/liquid).
Q.93 When H2S gas is passed through an aqueous solution of SO2 gas, a yellow turbidity is formed. Why?
Answer: On passing H2S gas through a solution of SO2, oxidation of H2S occurs. The particles of sulphur that are formed get condensed to be in the colloidal range. Therefore, the milky solution contains colloidal particles of sulphur.
2H2S+SO2→3S+2H2O (Colloidal)
Q.94 A reddish brown colloidal sol is formed when a small amount of FeCl3 is added to freshly precipitated Fe(OH)3. Explain.
Answer: Fe3+ ions present in the solution are adsorbed on Fe(OH)3. The positively charged particles formed mutually repel and get disintegrated resulting in a reddish brown colloidal sol.
Fe(OH)3(ppt.)+Fe3+(electrolyte)→[Fe(OH)3]Fe3+ Colloidalsol. This process of converting a freshly precipitated substance into colloidal solution is called peptisation.
Q.95 What is the basic difference between dialysis and osmosis?
Answer: The two processes appear to be same since both involve the diffusion through semi permeable membrane. However, in osmosis, only the solvent and not the solute particles can pass through the membrane but in dialysis, even small ions of the electrolyte can also pass through the membrane. But colloidal particles which are comparatively big in size cannot pass.
Q.96 2.56 g of sulphur present in 100 mL of solution in colloidal form shows an osmotic pressure of 2.463 atm at 300 K. How many atoms of sulphur get associated to form the colloidal sol?
Answer: Let us calculate the observed molar mass of sulphur in the colloidal solution with the help of Van't Hoff equation: 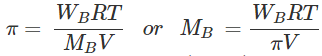
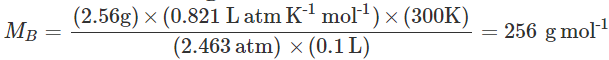
Gram atomic mass of sulphur =32gmol−1
∴ No. of sulphur atoms associated = 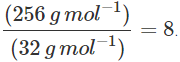
Q.97 Why do colloidal solutions differ in colours?
Answer: Colloidal solutions are generally coloured on account of the scattering of light by the particles of the dispersed phase i.e. colloidal particles. Since the particles present in different colloidal solutions differ in sizes, they impart specific colours to these solutions on passing light. The colour of the gold sol containing very fine particles of gold is red. As the particle size increases, the colour of solution changes to purple, blue and finally becomes golden yellow. As the particle size changes, the wavelength of the scattered light also changes resulting in different colours.
Q.98 The sol formed on adding silver nitrate solution to excess of potassium iodide solution is negatively charged while the same prepared in the reverse manner has positive charge. Explain.
Answer: The charge on the colloidal particles depends upon the preferential adsorption of the ions from the electrolyte that are common with their own lattice ions. When AgNO3 is added to an aqueous solution of KI, the yellow precipitate of AgI adsorbs I− ions from the electrolyte KI and forms negatively charged colloidal particles.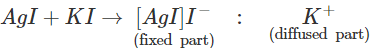 When formed on adding KI solution to an aqueous solution of AgNO3 positively charged Ag+ ions are adsorbed from the electrolyte and the colloidal particles acquire negative charge.
When formed on adding KI solution to an aqueous solution of AgNO3 positively charged Ag+ ions are adsorbed from the electrolyte and the colloidal particles acquire negative charge.
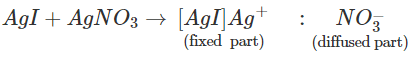
Q.99 There is a decrease in the extent of adsorption after sometime while absorption proceeds at a uniform rate. Explain.
Answer: Adsorption is a surface phenomenon. As more and more of the surface of the adsorbent gets occupied or covered, the extent of adsorption decreases. On the other hand, absorption occurs throughout the body of the absorbent. Therefore, it is not related to the surface and proceeds at a uniform rate.
Q.100 The gases which are liquefied more easily have a greater tendency to get adsorbed on the surface of the adsorbent than the gases which are liquefied with difficulty. Assign reason.
Answer: Gases which are liquefied more easily generally consist of polar molecules e.g. HCl,NH3,SO2 etc. This means that they have greater attraction towards the surface of the adsorbent. These are adsorbed more readily on the surface of the adsorbent as compared to gases like H2,He,N2 etc. which consist of non-polar molecules that have comparatively lesser attraction towards the surface of the adsorbent.
Q.101 Critical temperature of N2,CO and CH4 are 126, 134 and 190 K respectively. Arrange them in increasing order of adsorption on the surface of activated charcoal.
Answer: Critical temperature may be defined as the minimum temperature above which a gas cannot be liquefied however large its pressure may be. It may be noted that greater the critical temperature, easier will be the ease of liquefaction of the gas and more will be its extent of adsorption on a particular solid such as activated charcoal. Therefore the increasing order of the extent of adsorption is: N2<CO<CH4
Q.102 Gelatin is generally added to ice cream. Why?
Answer: Ice cream is an emulsion of milk or cream in water (oil in water). Gelatin is added in the preparation of ice cream to act as emulsifier i.e., it helps in the formation of a stable emulsion and as a result, ice cream can be preserved.
Q.103 The addition of ferric hydroxide sol to arseneous sulphide sol results in the precipitation of both. Explain.
Answer: This is possible only in case equimolar sols (solutions with same number of moles) are mixed. Since the ferric hydroxide sol carries positive charge and arseneous sulphide sol is negatively charged, on mixing they will get their charge neutralized and get coagulated or precipitated.
Q.104 Lyophilic sols are called reversible colloids. Assign reason.
Answer: Lyophilic sols are generally known as reversible colloids. In fact, if the dispersed phase is removed completely, the colloidal solution can be formed again by mixing the dispersed phase (residue) left with a fresh sample of dispersion medium. For example, if a colloidal sol of starch in water is dried completely, it can be reformed by mixing the residue with fresh water.
Q.105 Artificial rain can be caused by spraying charged dust particles over clouds. Discuss.
Answer: Clouds represent the colloidal solutions of water drops in air (liquid in gas type). These drops are expected to carry some charge (positive or negative). In order to neutralize the charge on these, charged dust particles carrying opposite charge are sprayed over a certain layer of cloud. These will neutralize the charge on water droplets resulting in their coagulation. The bigger water drops can no longer be retained by the atmosphere and will result in the artificial rain.
Q.106 Ferric hydroxide sol is more readily coagulated by Na3P04 in comparison to KCl. Why?
Answer: Ferric hydroxide sol is positively charged and to cause its coagulation, ions carrying negative charge are needed. Since PO3−4 ions have higher negative charge than Cl− ions therefore, Na3PO4 coagulates the ferric hydroxide sol most efficiently. This is according to Hardy-Schulze law. For details, consult Section 5.23.
Q.107 Delta is generally formed where river meets the ocean. How will you account for it?
Answer: River water is generally muddy and carries along with colloidal dust particles which are charged in nature. Sea water contains a large number of electrolytes. When the river comes in contact with the sea water, the colloidal particles get their charge neutralized by the oppositely charged ions present in sea water and are coagulated. This ultimately results a hard solid mass known as delta.
Q.108 The layer of fat in the pans used for manufacturing soaps can be removed by adding boiling washing soda solution. How will you account for it?
Answer: Washing soda (Na2CO3) gets hydrolysed to form NaOH as follows: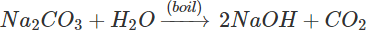 NaOH reacts with fat which is a triglyceride to form soap and glycerol by saponification reaction. The soap thus formed helps in cleaning the pan.
NaOH reacts with fat which is a triglyceride to form soap and glycerol by saponification reaction. The soap thus formed helps in cleaning the pan.
Q.109 Which out of the following solutions having the same concentration will be most effective in causing the coagulation of the arsenic sulphide sol that is yellow in colour: KCl,MgCl2,AlCl3 or Na3PO4?
Answer: Arsenic sulphide (As2S3) sol has a negative charge on it. To cause its coagulation or precepitation, the active ions must be positively charged. According to Hardy-SchuIze rule, greater the magnitude of the positive charge on the ion, more will be its coagulating power. Thus, AlCl3 containing Al3+ ions will be the most effective in causing the coagulation of the sol.
Q.110 100 mL of a standard sol requires 240 mg of starch for its protection against coagulation. Calculate gold number of starch.
Answer: Amount of starch required for 100 mL of gold sol = 240 mg Amount of starch required for 10 mL of gold sol = 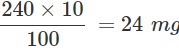 Therefore, gold number of starch = 24
Therefore, gold number of starch = 24
Q.111 Adsorption is always exothermic in nature ; Do you agree?
Answer: According to Gibb's Helmholtz equation: ΔG=ΔH−TΔS. Since entropy decreases as a result of adsorption,ΔS is -ve. Now, ΔG i.e., free energy change must be also -ve in case adsorption is to take place. At a given temperature, if ΔG is to be negative, then ΔH must be negative. This means that adsorption must be always exothermic in nature.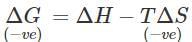
|
117 videos|226 docs|237 tests
|
FAQs on Short & Long Answer Questions: Surface Chemistry - 2 - Physical Chemistry for NEET
| 1. What is surface chemistry? |  |
| 2. What is adsorption in surface chemistry? |  |
| 3. What are the types of adsorption? |  |
| 4. How does surface area affect adsorption? |  |
| 5. What are the applications of surface chemistry in daily life? |  |





















