Short & Long Answer Questions: The d & f-Block Elements - 2 | Inorganic Chemistry for NEET PDF Download
Q.51 Write two important applications of potassium dichromate.
Answer: (i)Used as a primary standard in volumetric analysis.
(ii) Used as an oxidising agent in organic synthesis.
Q.52 What is the reason of the greater tendency of transition metals to form complex compounds?
Answer: Greater tendency of transition metals to form complexes is (i) due to their smaller sizes (ii) due to high ionic charges, (high charge/radius ratio) (iii) due to availability of vacant d-orbitals for bond formation.
Q.53 What are interstitial compounds? What are their main characteristics.
Answer: Interstitial compounds are those which are formed when small atoms like H, C or N are trapped inside the crystal lattices of metals. (i) They have high melting points, higher than those of pure metals. (ii) They are very hard. (iii) They retain metallic conductivities (iv) They are non stoichiometric in nature.
Q.54 Explain, why the chemistry of the actinoids is much men complicated?
Answer: The chemistry of actinoids is much more complicated because of its irregular electronic configuration.
Q.55 Discuss the consequences of lanthanoid contraction.
Answer: (i) Due to lanthanoid contraction the radii of elements of 4d and 5d series transition elements are almost same and hence they have similar physical properties. (ii) They occur in nature together. (iii) Their separation is difficult.
Q.56 What is actinoid contraction? What are the causes of actinoid contraction? Actinoid contraction has consequence. Why?
Answer: The gradual decrease in atomic radii from Actinium to Lawrencium is called Actinoid contraction. This contraction is greater from element to element than the lanthanoid contraction. There is no consequence of actinoid contraction because elements succeeding actinoids are much less known.
Q.57 The lanthanides resemble one another more closely. On reasons.
Answer: Lanthanoids resemble more closely with one another. Due to lanthanoid contraction the atomic radii of lanthanoids are almost similar and they show common +3 oxidation state. So, they are found in nature together and their physical properties are quite similar.
Q.58 Mn (II) shows maximum paramagnetic character amongst the divalent ions of the first transition series.
Answer: The configuration of Mn(II) ion: 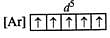 Since all the 3d orbitals are half-filled in Mn(II), it is maximum paramagnetic among the divalent ions of first transition series of elements.
Since all the 3d orbitals are half-filled in Mn(II), it is maximum paramagnetic among the divalent ions of first transition series of elements.
Q.59 The lowest oxidation state of manganese is basic while the highest is acidic.
Answer: The lowest oxidation state of manganese is  . It has a tendency to lose electrons in order to increase its oxidation state. Therefore,
. It has a tendency to lose electrons in order to increase its oxidation state. Therefore, 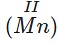 is basic. In highest oxidation state of +7(Mn)VII,, there is no scope for the loss of anymore electrons. It can rather accept the same. This shows that MnVII can act as acid by accepting electrons.
is basic. In highest oxidation state of +7(Mn)VII,, there is no scope for the loss of anymore electrons. It can rather accept the same. This shows that MnVII can act as acid by accepting electrons.
Q.60 Why do chromium group elements have the highest melting points in their respective series?
Answer: The elements belonging to this group. (Cr, Mo, W, Sg) have maximum number of unpaired d-electrons (d5 configuration). Therefore metallic bonding is the maximum and so are the melting points.
Q.61 Why do transition metals form coloured complexes?
Answer: Most of the compounds of transition elements are coloured due to the presence of unpaired electrons in d-sub shell of transition metal ions. It can be explained with the help of crystal field theory. For example, in an octahedral complex [Ti(H2O)6]3+ under the influence of ligands (H2O molecules), all the five degenerate d-orbitals of penultimate shell of transition metal ion Ti3+ split up in to two degenerate sets, one containing dx2−y2 and d2z (higher energy) and the other containing dxy,dyz,dzx (with lower energy). When white light falls, a part of it corresponding to a certain wavelength (yellow in this case) is absorbed. Due to small energy difference, electronic excitations take place from one set to another (d-d transition). The remaining colours of white light (red and blue) are transmitted and the compound appears purple coloured.
Q.62 Among transition metals, the highest oxidation state is exhibited in oxoanions of a metal. Explain.
Answer: The oxoanions of metals have covalent nature in which oxygen is involved in the multiple bonding with the metal atom. This is responsible for the high oxidation state of the metal in oxoanions. For example, the elements Mn exhibits +7 oxidation state in permanganate ion (MnO4)−.
Q.63 Ce4+ is used as an oxidising agent in volumetric analysis. Why?
Answer: Ce4+ has the noble configuration of Xe (Z = 54) but it has a strong urge to change to + 3 oxidation state which is the most common oxidation state of the elements belonging to lanthanoid family. Therefore, it acts as a strong oxidizing agent in the volumetric analysis.
Q.64 Zn2+ salts are white while Cu2+ salts are blue.
Answer: Cu2+ ion (3d9) has one half filled d-orbital. Therefore, it has an urge to take part in d?d transition. The compounds containing Cu2+ ion (e.g., CuSO4.5H2O) are coloured. Zn2+ ion (3d10) has completely filled d-orbitals and there is no scope for any electron transition. Therefore, Zn2+ salts are white and not coloured.
Q.65 Zr and Hf have identical sizes.
Answer: Both Zr and Hf are present in group 4 (d-block). The atomic radius of Zr (160 pm) is quite close to that of Hf (159 pm). Actually, Hf experiences lanthanoid contraction which causes a decrease in its atomic size and atomic radius. Therefore, the two elements have identical sizes. For more details, consult section 8.11.
Q.66 Among the lanthanoids, Ce (III) is easily oxidised to Ce (IV). Discuss.
Answer: The element cerium (Ce) with atomic number (Z = 58) has the electronic configuration [Xe]4f15d16s2.Ce(III) ion formed by losing three electrons has the configuration [Xe]4f1. The ion can lose another electron readily to form Ce(IV)ion with configuration of noble gas xenon (Xe).
Q.67 Copper (I) has d10 configuration while copper (II) has d9 configuration, still copper (II) is more stable in aqueous solution than copper (I). Assign reason.
Answer: Cu+ion has more symmetrical configuration as compared to Cu2+ ion. It is therefore, expected to be more stable. However, in aqueous solution Cu2+ ion and the corresponding compounds are more stable because of the smaller size of Cu2+ ion and its greater tendency to get hydrated as compared to Cu+ ion.
Q.68 With 3d4 configuration, Cr2+ acts as a reducing agent but Mn3+ acts as an oxidising agent. Explain.
Answer: Cr2+(3d4)→Cr3+(3d3)+e−; Mn3+(3d4)+e−→Mn2+(3d5) Cr2+ acts as a reducing agent due to greater stability of Cr3+ with exactly half-filled t2g level. Mn3+ acts as an oxidising agent due to extra stability of Mn2+ ion with all half filled d-orbitals.
Q.69 The element scandium (Z = 21) does not exhibit variable oxidation states and yet it is regarded as a transition element. Explain
Answer: The electronic configuration of Sc (Z = 21); Sc(Z=21):[Ar]184s23d1. The element exhibits +3 oxidation state in its compounds because by losing the three electrons, it acquires a noble gas configuration. However, it is still regarded as a transition element because it has a partially filled d sub-shell.
Q.70 E° value for the Mn3+/Mn2+ couple is much more positive than for Cr3+/Cr2+ couple. Assign reason.
Answer: Mn(Z=25):[Ar]184s23d5
Cr(Z=24):[Ar]184s13d5
Mn2+:[Ar]183d5
Cr2+:[Ar]183d4
Mn3+:[Ar]183d4
Cr3+:[Ar]183d3.
The electronic configuration of Mn2+ ion is more symmetrical as compared to that of Cr2+ ion. Therefore, third ionisation potential of Mn2+is much higher. Consequently E° value for the Mn3+/Mn2+ couple is much more positive than for Cr3+/Cr2+ couple.
Q.71 Why is chemistry of all lanthanoids identical?
Answer: In the lanthanoid family, filling of electrons takes place (n−2)f−orbitals i.e., 4f orbitals whereas they actually belong to sixth period (n = 6). This means that these 4/electrons are shielded by the electrons present in the outer shells and are normally not available for the bond formation. Therefore, chemistry of all lanthanoids (similarly of actinoids) is identical. For more details, consult Section 8.4.
Q.72 On the basis of the standard electrode potential values stated for acid solution, predict whether,Ti4+ species may be used to oxidise FeII to FeIII. Given Ti4++e−→Ti3+;E⊙−=+0.01V;Fe3++e−→Fe2+;E⊙−=+0.77V.
Answer: The E⊙− (standard reduction potential) values show that FeII ions can be oxidised to FeIII ions by accepting electrons from Ti3+ ion. However, the reverse reaction i.e., oxidation of Fe11 to FeIII is not possible.
Q.73 Out of Fe2+ and Fe3+ ions, which is more stable?
Answer: Fe3+ ion is more stable than Fe2+ ion. This is explained on the basis of the electronic configurations of the two ions. Fe3+ ion with all the five 3d orbitals half filled, is more symmetrical than Fe2+ ion in which four 3d orbitals are half filled and one is filled. Therefore, Fe3+ ion is more stable than Fe2+ ion. Fe2+:1s22s22p63s23p63d6;Fe3+:1s22s22p63s23p63d5 Moreover, Fe3+ ion has a greater tendency to get hydrated in aqueous solution as compared to Fe2+ ion. It is therefore, expected to be more stable.
Q.74 Anhydrous ferric chloride cannot be obtained by heating hydrated ferric chloride. Discuss.
Answer: Hydrated ferric chloride (FeCl3.6H2O) upon heating gets hydrolysed by its own molecules of water of crystallization to give ferric hydroxide which changes to ferric oxide on further heating. Thus, anhydrous ferric chloride is not formed. FeCl3.6H2O→Fe(OH)3+3HCl+3H2O]×2 2Fe(OH)3→Fe2O3+3H2O 2FeCl3.6H2O→Fe2O3+6HCl+9H2O
Q.75 Why is Cu (I) diamagnetic while Cu (II) is paramagnetic?
Answer: The configuration of copper in the two oxidation states is: Misplaced &. Cu (I) has all filled orbitals and is diamagnetic in nature. On the other hand, Cu (II) has one half-filled orbital and is paramagnetic in nature.
Q.76 Zn2+ salts are white while Cu2+ salts are blue. Explain.
Answer: The Zn2+ ion has all filled orbitals whereas Cu2+ ion (3d9 configuration) has one half filled 3d-orbital. It has therefore, a tendency to form coloured ion in solution whereas Zn2+ has no such tendency. Thus, Zn2+ salts are white while those of Cu2+ are blue.
Q.77 Cu+ is a d10 ion and colourless whereas Cu2O is red and Cu2S is black. Explain.
Answer: In Cu+ ion (d10), there is nod−d∗transition and the ion is colourless. Both Cu2O and Cu2S are coloured because of charge transfer of electrons from O2− or S2− to the vacant orbital of Cu+ ion.
Q.78 Out of Co2+ and Ni2+ ions, which has lower magnetic moment?
Answer: It may be noted that the magnetic moment is linked with the number of unpaired electrons present in an ion. Greater the number of such electrons, more will be magnetic moment. Configuration of both Co2+ and Ni2+ ions are given as: Co2+(Z=27);[Ar]183d7;Ni2+(Z=28);[Ar]183d8. Since the number of unpaired electrons in Co2+ ion is more than in Ni2+ ion it has therefore, more magnetic moment than Ni2+ ion.
Q.79 Why is ZnO yellow when hot and white when cold?
Answer: Zn2+ ion (ZnO) has 3d10 electronic configuration with all the electrons paired. Therefore, ZnO is white. The yellow colour of ZnO is due to non-stoichiometric defect leading to extra cation at the interstitial sites. For details, consult Section 8.4.
Q.80 Transition elements form a number of interstitial compounds. Explain.
Answer: In the crystal structures of transition metals, small vacant sites known as interstitial sites are present. These are occupied by atoms of smaller elements such as carbon, hydrogen, nitrogen, boron etc. This leads to the formation of compounds known as interstitial compounds. The properties of these metals are somewhat different than the pure metals. For example, compound consisting of iron and carbon is harder than iron. Generally these compounds are less malleable and ductile but at the same time have greater tensile strength than pure metals. For more details, consult Section 8.4.
Q.81 Chromium is a typical hard metal while mercury is a liquid. Explain.
Answer: In chromium, the inter atomic bonding in the atoms is very strong because they have five unpaired electrons (3d54s1) in the d-subshell. Therefore, chromium is a very hard metal. On the contrary, in mercury, the atoms have fully filled d-orbitals (5d106s2) and the bonding in them is quite weak. Therefore, mercury is a liquid at room temperature.
Q.82 Transition metals of 3d-series do not react readily with dilute acids to liberate hydrogen although they have high negative reduction potential values. Assign reason.
Answer: These are expected to liberate hydrogen from dilute acids due to their reducing nature as indicated by E° values. But most of them react very slowly because of the formation of the protective coating of their oxides on the surface. This coating is rather inert and does allow the metals to react with dilute acids so readily to evolve hydrogen gas.
Q.83 The second and third members in each group of the transition elements have similar metallic radii. Assign reason.
Answer: This is because of lanthanoid contraction in the members of the lanthanoid family (Z = 58 to Z = 71) which occupy a position along with Lanthanum (Z = 57) in the periodic table. However, these are placed separately at the bottom of the table in the f-block. The lanthanoid contraction also decreases the size of the elements of the third transition series (Hf onwards). As a result, there is a negligible difference in the metallic radii of the second and third members in each group of transition metals. For more details, consult Section 8.4.
Q.84 The atomic radii of the elements in a transition series do not vary much while they do vary in case of s and p block elements. Explain.
Answer: In case of transition elements, the electrons are filled in (n−1) d-sub-shell and they exert screening effect as a result of which the atomic size is expected to increase. Thus, as we move from left to the right in a transition series, the increase in the magnitude of nuclear charge (leading to decrease in size) is almost balanced by the increase in the shielding effect (leading to increase in size). Because of these two opposing tendencies, there is a very small change in the atomic size (or atomic radii) as we move along the transition series. In s and p-block elements, as we move along a period the electrons are filled in the valence s and p orbitals. These electrons do not exert as much screening effect as are exerted by the electrons present in the d-orbitals. There is a gradual increase in effective nuclear change leading to decrease in atomic size along a particular period.
|
74 videos|106 docs|111 tests
|
FAQs on Short & Long Answer Questions: The d & f-Block Elements - 2 - Inorganic Chemistry for NEET
| 1. What are the d-block and f-block elements? |  |
| 2. What are the properties of d-block elements? |  |
| 3. What is the significance of d-block elements? |  |
| 4. What are the electronic configurations of d-block elements? |  |
| 5. What are the properties of f-block elements? |  |

|
Explore Courses for NEET exam
|

|

















