Class 10 Science Chapter 2 Question Answers - Acids, Bases and Salts
Short Q&A:
Q1: Explain why an antacid tablet is taken when we suffer from acidity.
Ans : Sometimes when we consume spicy food, our stomach releases excess of hydrochloric acid which causes indigestion or acidity. An antacid tablets contains base like milk of magnesia (magnesium hydroxide), it neutralises the effect of excessive acid and bring reliefs.
Q2: Red litmus paper is dipped in a solution; it remains red, what is the nature of the solution?
Ans : Red litmus paper when dipped in a solution, if it remains red then the nature of the solution is neutral.
Q3: What are salts? Give examples.
Ans : Salts are the ionic compounds generally formed by neutralisation of an acid with base. They can be acidic, basic as well as neutral. Example acidic salts: sodium bicarbonate, basic salts: magnesium chloride, neutral salt: sodium chloride.
Q4: Give examples of some acids and bases
Ans : Curd, lemon juice, vinegar, orange juice etc. are acids and baking soda, lime water etc. are bases.
Q5: Define indicators along with examples.
Ans : Indicators are special type of substance that are used to taste whether a substance is acidic or basic in nature. It change the colour of acidic or basis substances when added into it. Turmeric, litmus, etc. are some natural indicators.
Q6: In distilled water , litmus paper turns into _________
Ans : Litmus is extracted from lichens. It is most commonly used as an indicator to determine the chemical nature of substance. It has mauve or purple colour in distilled water. When it is added to an acidic solution, it turns red and when added to a basic solution, it turns blue. It is available in the form of a solution, or in the form of strips of paper, known as litmus paper. Generally, it is available as red and blue litmus paper.
Q7: What is the use of litmus test?
Ans : To test the nature of a substance for an Acid or a base or a neutral, litmus test is performed in which the Acid turns blue litmus red, Bases turn red litmus blue and has no effect on neutral substance.
Q8: What are the effects of acid rain?
Ans : Effects of Acid Rain:
- Makes water bodies acidic, harming aquatic life.
- Damages leaves of trees and crop plants.
- Corrodes metal structures like steel bridges.
- Erodes surfaces of buildings and monuments, especially marble.
Q9: What do you mean by neutral substance, explain with examples?
Ans : The substances which are neither acidic, nor basic are called neutral substance. These substances neither turn blue litmus red nor red litmus blue, for example distilled water, sugar solution etc.
Q10: Rena is trying to wash turmeric stain on her cloth with soap, she noticed the stain colour changed to red, explain why?
Ans : Turmeric is a natural indicator which when reacts with bases turns it into red colour; here soap solution is basic so it turns red.
Q11: A shopkeeper has a few bottles of soft drink in his shop. But, unfortunately, these are not labelled. He has to serve the drinks on the demand of customers. One customer wants acidic drink; another wants basic and third one wants neutral drink. How will he decide which drink is to be served to whom?
Ans : A shopkeeper can identify which soft drink is acidic, basic, or neutral by using simple tests based on the properties of acids and bases. Acidic drinks have a sour taste and will turn blue litmus paper red. Basic drinks, on the other hand, turn red litmus paper blue and usually taste bitter. Neutral drinks do not change the colour of either red or blue litmus paper and have no sour or bitter taste. Besides litmus paper, turmeric solution can also be used as a test because it changes colour to reddish-brown in the presence of a base but remains yellow in acidic and neutral drinks. Using these tests, the shopkeeper can serve the correct drink to each customer based on their demand.
Q12: Arnav is provided with three kinds of liquid of them one is sodium hydroxide; another is hydrochloric acid and third is a sugar solution. How will he identify them when he have only turmeric indicator.
Ans : Turmeric is yellow in colour, when it is exposed to neutral (Sugar Solution) or acidic substances (Hydrochloric Acid) it will retain its yellow colouration. However, if turmeric is exposed to more alkaline substances (sodium hydroxide) it becomes a dark pink/red. So first we detect sodium hydroxide -a basic substance by a colour change from yellow to dark or red. Then will test for an acid or neutral substance with indication of no colour change. Now out of these two, we will mix one with already tested solution for basic substance -sodium hydroxide with dark or red colour, if on mixing the colour reverses back to yellow, the liquid is an acid and the remaining third liquid is neutral.
Q13: Explain why factory waste should be neutralised before disposing it into the water bodies.
Ans : The wastes of many factories contain acids. If they are allowed to flow into the water bodies, the acids will kill fish and other organisms. The factory wastes are, therefore, neutralised by adding basic substances into it.
Q14: Why Calamine solution is applied on the skin when an ant bites?
Ans : The sting of an ant contains formic acid. When an ant bites, it injects the acidic liquid into the skin. The effect of the sting can be neutralised by rubbing some moist solution of a basic substance such as baking soda (sodium hydrogen carbonate) or calamine solution, which contains zinc carbonate.
Q15: What is acid rain ?
Ans: Acid rain contains excess acids formed when carbon dioxide, sulfur dioxide, and nitrogen dioxide dissolve in rainwater to form carbonic acid, sulfuric acid, and nitric acid. It causes damage to buildings, plants, animals, and historical monuments.
Long Q&A:
Q1: State few properties of acids.
Ans : Acids are substances known for their sour taste (though tasting them is not safe). The word "acid" is derived from the Latin word 'ACERE', meaning sour.
Properties of Acids:
- Sour in taste.
- Turn blue litmus paper red.
- Turn China rose solution dark pink or magenta.
Examples of acidic substances include curd, vinegar, lemon, and orange juice, which all contain natural acids.
 Citrus Fruits are Acidic in Nature
Citrus Fruits are Acidic in Nature
Q2: State few properties of bases.
Ans : Bases are substances with a bitter taste and a slippery feel.
Properties of Bases:
- Bitter in taste.
- Turn red litmus paper blue.
- Turn turmeric paper reddish-brown.
- Turn China rose solution green.
Some common examples of bases include baking soda, milk of magnesia, and soap. The chemical nature of these substances is referred to as basic.
Q3: What are the differences between acids and bases?
Ans : 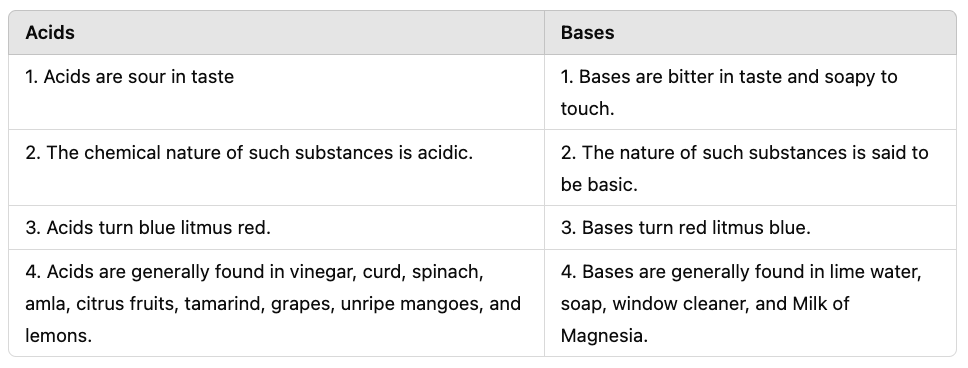
Q4: Describe the process of neutralisation with the help of an example.
Ans :
Neutralisation is a process in which an acid solution when mixed with base solution, react with each other to produce a salt and water along with generation of heat. Salt so produced, may be acidic, basic or neutral in nature. In this process the acidic nature of the acid and the basic nature of the base are destroyed.
Acid + base → salt + water. (heat is evolved)
For example: HCl + NaOH→ NaCl + H2O.
Hydrochloric acid + Sodiumhydroxide → Sodium chloride + Water
Q5: Explain the process and treatment of an ant bite.
Ans: When an ant bites, it injects an acidic liquid called formic acid into the skin, causing burning pain, redness, and irritation. The effect of the acid can be neutralized by applying a mild base to the affected area.
Two common treatments are:
Baking soda solution: Baking soda (sodium hydrogen carbonate) neutralizes the acid and reduces the pain and irritation.
Calamine solution: Calamine contains zinc carbonate, which also neutralizes the acidic effect and provides relief.
Applying either of these substances helps neutralize the acid and reduce the discomfort caused by the ant bite.
|
112 videos|286 docs|28 tests
|
FAQs on Class 10 Science Chapter 2 Question Answers - Acids, Bases and Salts
| 1. What are the properties of acids and bases? |  |
| 2. How do we identify acids and bases using indicators? |  |
| 3. What is the role of salts in everyday life? |  |
| 4. What is the difference between strong and weak acids? |  |
| 5. How do neutralization reactions work? |  |

















