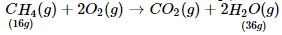Assertion & Reason Type Questions: Some Basic Concepts of Chemistry | Physical Chemistry for NEET PDF Download
Question 1:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion: One atomic mass unit is defined as one twelfth of the mass of one carbon – 12 atom.
Reason: Carbon-12 isotope is the most abundant isotope of carbon and has been chosen as standard.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (b)
Question 2:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion: Equal moles of different substances contain same number of constituent particles.
Reason: Equal weights of different substances contain the same number of constituent particles.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (c)
Equal moles of different substances contain same number of constituent particles but equal weights of different substances do not contain the same number of constituent particles.
Question 3:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion (A): combustion of 16 g of methane gives 18g of water.
Reason (R): In the combustion of methane, water is one of the products.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (c)
Combustion of 16 g of methane gives 36 g of water.
Question 4:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion (A): One atomic mass unit is defined as one twelfth of the mass of one carbon-12 atom.
Reason (R): Carbon-12 isotope is the most abundant isotope of carbon and has been chosen as standard.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (b)
Both A and R are true but R is not the correct explanation of A.
Question 5:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion: The empirical mass of ethene is half of its molecular mass.
Reason: The empirical formula represents the simplest whole number ratio of various atoms present in a compound.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (a)
Question 6:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion (A): The empirical mass of Ethene is half of its molecular mass.
Reason (R): The empirical formula represents the simplest whole number ratio of various atoms present in a compound.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (a)
Question 7:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion: Volume of a gas is inversely proportional to the number of moles of gas.
Reason: The ratio by volume of gaseous reactants and products is in agreement with their mole ratio.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (d)
Question 8:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion (A): Significant figures for 0.200 is 3 where as for 200 it is 1.
Reason (R) Zero at the end or right of a number are significant provided they
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (c)
Zero at the end or right of a number are significant provided they are on the right side of the decimal point.
Question 9:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion: Significant figures for 0.200 is 3 whereas for 200 it is 1.
Reason Zero at the end or right of a number are significant provided they are not on the right side of the decimal point.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (c)
Question 10:
Directions: In the following questions a statement of assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choice given below.
Assertion : 1.231 has three significant figures.
Reason : All numbers right to the decimal point are significant.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Correct answer is option (d)
1.231 has four significant figures all no. from left to right are counted, starting with the first digit that is not zero for calculating the no. of significant figure.
|
117 videos|225 docs|239 tests
|
FAQs on Assertion & Reason Type Questions: Some Basic Concepts of Chemistry - Physical Chemistry for NEET
| 1. What are some basic concepts of chemistry? |  |
| 2. What is the difference between an atom and a molecule? |  |
| 3. What are elements and compounds in chemistry? |  |
| 4. What are chemical reactions? |  |
| 5. How do basic concepts of chemistry apply to everyday life? |  |

|
Explore Courses for NEET exam
|

|