UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Step-Growth Polymers—Condensation Polymers
Step-Growth Polymers—Condensation Polymers | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Condensation Polymers |

|
| Characteristics of Condensation Polymers |

|
| What are polyamides? |

|
| What is a polyester? |

|
| Making polyesters as an example of condensation polymerisation |

|
Condensation Polymers
- A large number of important and useful polymeric materials are not formed by chain-growth processes involving reactive species such as radicals, but proceed instead by conventional functional group transformations of polyfunctional reactants. These polymerizations often (but not always) occur with loss of a small byproduct, such as water, and generally (but not always) combine two different components in an alternating structure.
- The polyester Dacron and the polyamide Nylon 66, shown here, are two examples of synthetic condensation polymers, also known as step-growth polymers. In contrast to chain-growth polymers, most of which grow by carbon-carbon bond formation, step-growth polymers generally grow by carbon-heteroatom bond formation (C-O & C-N in Dacron & Nylon respectively). Although polymers of this kind might be considered to be alternating copolymers, the repeating monomeric unit is usually defined as a combined moiety.
- Examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly(β-hydroxybutyric acid), a polyester synthesized in large quantity by certain soil and water bacteria. Formulas for these will be displayed below by clicking on the diagram.

Characteristics of Condensation Polymers
- Condensation polymers form more slowly than addition polymers, often requiring heat, and they are generally lower in molecular weight. The terminal functional groups on a chain remain active, so that groups of shorter chains combine into longer chains in the late stages of polymerization. The presence of polar functional groups on the chains often enhances chain-chain attractions, particularly if these involve hydrogen bonding, and thereby crystallinity and tensile strength. The following examples of condensation polymers are illustrative.
- Note that for commercial synthesis the carboxylic acid components may actually be employed in the form of derivatives such as simple esters. Also, the polymerization reactions for Nylon 6 and Spandex do not proceed by elimination of water or other small molecules. Nevertheless, the polymer clearly forms by a step-growth process. Some Condensation Polymers.

- The difference in Tg and Tm between the first polyester (completely aliphatic) and the two nylon polyamides (5th & 6th entries) shows the effect of intra-chain hydrogen bonding on crystallinity. The replacement of flexible alkylidene links with rigid benzene rings also stiffens the polymer chain, leading to increased crystalline character, as demonstrated for polyesters (entries 1, 2 &3) and polyamides (entries 5, 6, 7 & 8). The high Tg and Tm values for the amorphous polymer Lexan are consistent with its brilliant transparency and glass-like rigidity. Kevlar and Nomex are extremely tough and resistant materials, which find use in bullet-proof vests and fire resistant clothing.

- Many polymers, both addition and condensation, are used as fibers The chief methods of spinning synthetic polymers into fibers are from melts or viscous solutions. Polyesters, polyamides and polyolefins are usually spun from melts, provided the Tm is not too high. Polyacrylates suffer thermal degradation and are therefore spun from solution in a volatile solvent. Cold-drawing is an important physical treatment that improves the strength and appearance of these polymer fibers.
- At temperatures above Tg, a thicker than desired fiber can be forcibly stretched to many times its length; and in so doing the polymer chains become untangled, and tend to align in a parallel fashion. This cold-drawing procedure organizes randomly oriented crystalline domains, and also aligns amorphous domains so they become more crystalline. In these cases, the physically oriented morphology is stabilized and retained in the final product. This contrasts with elastomeric polymers, for which the stretched or aligned morphology is unstable relative to the amorphous random coil morphology.
- This cold-drawing treatment may also be used to treat polymer films (e.g. Mylar & Saran) as well as fibers.

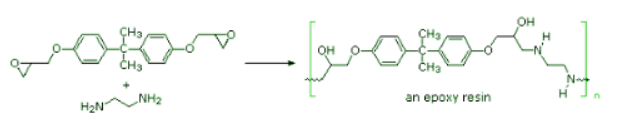
- Step-growth polymerization is also used for preparing a class of adhesives and amorphous solids called epoxy resins. Here the covalent bonding occurs by an SN2 reaction between a nucleophile, usually an amine, and a terminal epoxide. In the following example, the same bisphenol A intermediate used as a monomer for Lexan serves as a difunctional scaffold to which the epoxide rings are attached. Bisphenol A is prepared by the acid-catalyzed condensation of acetone with phenol.

Question for Step-Growth Polymers—Condensation PolymersTry yourself: Which type of polymers generally grow by carbon-heteroatom bond formation?View Solution
What are polyamides?
- Polyamides are polymers where the repeating units are held together by amide links. An amide group has the formula - CONH2. An amide link has this structure:

- In an amide itself, of course, the bond on the right is attached to a hydrogen atom.
Nylon
- In nylon, the repeating units contain chains of carbon atoms. (That is different from Kevlar, where the repeating units contain benzene rings - see below.) There are various different types of nylon depending on the nature of those chains.
Nylon-6,6
- Nylon-6,6 is made from two monomers each of which contain 6 carbon atoms - hence its name. One of the monomers is a 6 carbon acid with a -COOH group at each end - hexanedioic acid. The other monomer is a 6 carbon chain with an amino group, -NH2, at each end. This is 1,6-diaminohexane (also known as hexane-1,6-diamine).

- When these two compounds polymerise, the amine and acid groups combine, each time with the loss of a molecule of water. This is known as condensation polymerization. Condensation polymerization is the formation of a polymer involving the loss of a small molecule. In this case, the molecule is water, but in other cases different small molecules might be lost.
- The diagram shows the loss of water between two of the monomers:

- This keeps on happening, and so you get a chain which looks like this:

Nylon-6
- Iit is possible to get a polyamide from a single monomer. Nylon-6 is made from a monomer called caprolactam.

- Notice that this already contains an amide link. When this molecule polymerizes, the ring opens, and the molecules join up in a continuous chain.

Kevlar
- Kevlar is similar in structure to nylon-6,6 except that instead of the amide links joining chains of carbon atoms together, they join benzene rings. The two monomers are benzene-1,4-dicarboxylic acid and 1,4-diaminobenzene.

- If you line these up and remove water between the -COOH and -NH2 groups in the same way as we did with nylon-6,6, you get the structure of Kevlar:

What is a polyester?
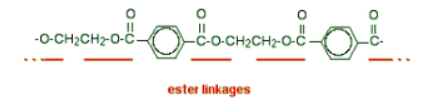
- A polyester is a polymer (a chain of repeating units) where the individual units are held together by ester linkages.

- The diagram shows a very small bit of the polymer chain and looks pretty complicated. But it is not very difficult to work out - and that's the best thing to do: work it out, not try to remember it. You will see how to do that in a moment.
- The usual name of this common polyester is poly(ethylene terephthalate). The everyday name depends on whether it is being used as a fibre or as a material for making things like bottles for soft drinks. When it is being used as a fiber to make clothes, it is often just called polyester. It may sometimes be known by a brand name like Terylene. When it is being used to make bottles, for example, it is usually called PET.
Question for Step-Growth Polymers—Condensation PolymersTry yourself: Which type of polymerization process is used to produce polyesters?View Solution
Making polyesters as an example of condensation polymerisation
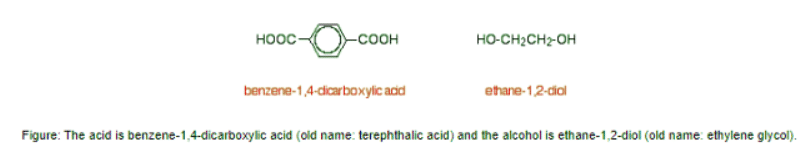
- In condensation polymerisation, when the monomers join together a small molecule gets lost. That's different from addition polymerisation which produces polymers like poly(ethene) - in that case, nothing is lost when the monomers join together. A polyester is made by a reaction involving an acid with two -COOH groups, and an alcohol with two -OH groups. In the common polyester drawn below.

- Now imagine lining these up alternately and making esters with each acid group and each alcohol group, losing a molecule of water every time an ester linkage is made.

- That would produce the chain shown above (although this time written without separating out the carbon-oxygen double bond - write it whichever way you like).

The document Step-Growth Polymers—Condensation Polymers | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Step-Growth Polymers—Condensation Polymers - Chemistry Optional Notes for UPSC
| 1. What are the characteristics of condensation polymers? |  |
Ans. Condensation polymers are formed through a condensation reaction between two different monomers. They have the following characteristics:
- They are formed by the elimination of a small molecule, such as water or alcohol, during the polymerization process.
- They have repeating units that are connected by covalent bonds.
- They typically have high molecular weights and are often solid at room temperature.
- They can be biodegradable and environmentally friendly, depending on the monomers used.
- They exhibit a wide range of physical and chemical properties, making them suitable for various applications.
| 2. What are polyamides? |  |
Ans. Polyamides are a type of condensation polymer that contain amide groups (-CONH-) in their polymer chain. They are commonly known as nylon and are widely used in textiles, engineering plastics, and other applications. Polyamides exhibit high strength, excellent abrasion resistance, and good thermal stability. They can be easily molded into various shapes and have a wide range of chemical resistances. Polyamides are often used in the production of fibers, films, ropes, and automotive parts.
| 3. What is a polyester? |  |
Ans. A polyester is a type of condensation polymer that contains ester functional groups (-COO-) in its polymer chain. It is formed by the condensation reaction between a dicarboxylic acid and a diol. Polyesters have a wide range of applications, including textiles, packaging materials, and electrical insulation. They exhibit excellent resistance to chemicals, UV radiation, and moisture. Polyesters can be easily dyed, have good dimensional stability, and can be processed into various forms such as fibers, films, and bottles.
| 4. How are polyesters made as an example of condensation polymerization? |  |
Ans. Polyesters are made through condensation polymerization, which involves the reaction between a dicarboxylic acid and a diol. The reaction occurs in the presence of a catalyst and involves the elimination of a small molecule, usually water. The carboxylic acid and diol molecules react to form ester linkages (-COO-) between them, resulting in the formation of the polyester polymer. The process can be carried out using different methods such as melt polymerization, solution polymerization, or interfacial polymerization, depending on the desired product and processing conditions.
| 5. What are step-growth polymers and how are they related to condensation polymers? |  |
Ans. Step-growth polymers, also known as condensation polymers, are formed through a stepwise reaction between two or more monomers. In this type of polymerization, each monomer contains functional groups that can react with each other, resulting in the formation of covalent bonds and the elimination of small molecules. Condensation polymers, including polyamides and polyesters, are examples of step-growth polymers. They differ from addition polymers, such as polyethylene or polypropylene, where the polymer chains grow through the repetitive addition of monomers without the elimination of small molecules.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches