Stress Physiology | Botany Optional for UPSC PDF Download
Introduction
- In both natural and agricultural settings, plants frequently encounter various environmental stresses. Some of these stressors, such as rapid changes in air temperature, can become problematic in a matter of minutes, while others, like soil water content fluctuations or mineral deficiencies, may take days, weeks, or even months to adversely affect plants. It has been estimated that due to suboptimal climatic and soil conditions (abiotic factors), field-grown crops in the United States only achieve 22% of their genetic potential yield (Boyer 1982).
- Furthermore, stress plays a significant role in defining the range of plant species in different ecosystems, making it essential to understand the physiological processes underlying stress damage and how plants adapt and acclimate to these environmental challenges. This knowledge is crucial for both agriculture and environmental management.
- The term "plant stress" is sometimes used ambiguously, and the associated terminology can be confusing. To clarify, stress is typically described as an external factor that negatively impacts plant health. This chapter primarily focuses on environmental or abiotic factors that induce stress in plants, although biotic factors like weeds, pathogens, and insect predation can also contribute to stress. In most cases, stress is evaluated concerning plant survival, crop yield, growth (biomass accumulation), or fundamental assimilation processes like CO2 and mineral uptake, which are linked to overall growth.
- The concept of stress is closely related to stress tolerance, which refers to a plant's ability to thrive in unfavorable conditions. While "stress resistance" is sometimes used interchangeably with stress tolerance, the latter term is preferred. It's important to note that what is considered a stressful environment for one plant may not be so for another. For instance, peas (Pisum sativum) and soybeans (Glycine max) thrive at different temperatures, with peas experiencing heat stress sooner than soybeans. Consequently, soybeans exhibit greater heat stress tolerance.
- When a plant becomes more tolerant to stress due to prior exposure, it is said to be acclimated or hardened. Acclimation should not be confused with adaptation, which usually refers to a genetically determined resistance acquired through selection over many generations. Unfortunately, the term "adaptation" is sometimes used in the literature to describe acclimation. Furthermore, gene expression plays a significant role in acclimation.
- Both adaptation and acclimation to environmental stressors result from coordinated processes occurring at various organizational levels, from anatomy and morphology to cellular, biochemical, and molecular levels. For example, when faced with water deficit, plants may respond by wilting leaves, which reduces water loss and exposure to light, thereby mitigating heat stress on leaves.
- At the cellular level, responses to stress encompass changes in the cell cycle, cell division, the endomembrane system, cell vacuolization, and cell wall architecture—all of which enhance cell tolerance to stress. Biochemically, plants modify their metabolism in several ways to cope with environmental stresses, including the production of osmoregulatory compounds like proline and glycine betaine. The molecular mechanisms linking stress signal perception to genomic responses leading to tolerance have been intensively studied in recent years.
- In this, we will explore these principles and examine how plants adapt and acclimate to various stressors, including water deficit, salinity, chilling and freezing, heat, and oxygen deficiency in the root zone. Air pollution, which is a significant source of plant stress, will be discussed in Web Essay 25.1. While it is convenient to study each stress factor separately, many of them are interconnected, and common cellular, biochemical, and molecular responses underlie many acclimation and adaptation processes. For example, water deficit often goes hand in hand with salinity in the root zone and heat stress in leaves. Furthermore, plants frequently display cross-tolerance, implying that resistance mechanisms to different stresses share many common features.
Water Deficit and Drought Resistance
- In this section, we will explore various mechanisms related to coping with water deficits and drought resistance, categorizing them into different types. Initially, we can differentiate between two aspects: desiccation postponement (the ability to maintain tissue hydration) and desiccation tolerance (the ability to function even when dehydrated). These are sometimes referred to as drought tolerance under high and low water conditions, respectively. In older literature, the term "drought avoidance" was often used instead of "drought tolerance," but this terminology is not entirely accurate because all surviving plants tolerate drought to some extent and cannot truly avoid it, as drought is a meteorological condition. Another category is "drought escape," which includes plants that complete their life cycles during the wet season before the onset of drought. These are the only plants that genuinely "avoid" drought.
- Within the desiccation postponement category, there are two subgroups: water savers and water spenders. Water savers use water conservatively, preserving some in the soil for later stages of their life cycle. On the other hand, water spenders aggressively consume water, often using large quantities of it. An example of a water spender is the mesquite tree (Prosopis sp.), which has deeply rooted systems and has caused significant damage to semi-arid rangelands in the southwestern United States due to its excessive water consumption. This has prevented the reestablishment of grasses with agricultural value.
Drought Resistance Strategies Vary with Climatic or Soil Conditions
- Drought resistance strategies employed by plants can vary depending on the specific climatic and soil conditions they encounter. The ability of a plant to thrive in water-limited environments, as outlined in Table 25.1, is influenced by the total available water and the plant's water-use efficiency, which is discussed further in Chapters 4 and 9. Plants that can either access more water or use water more efficiently are better equipped to withstand drought. Some plants have adaptations, like the C4 and CAM photosynthesis pathways, enabling them to thrive in arid regions. Additionally, plants possess mechanisms that help them acclimate to water stress.

- Water deficit can be described as any condition in which the water content of a tissue or cell falls below the highest water content observed when it is fully hydrated. When water deficit occurs gradually enough to allow for adjustments in developmental processes, it affects growth in several ways, one of which is limiting leaf expansion. Leaf size is crucial because it typically correlates with photosynthesis rates. Nevertheless, rapid leaf expansion can deplete water resources too quickly.
- If precipitation primarily occurs in winter and spring, with dry summers, accelerated early growth can result in large leaf areas, rapid water consumption, and insufficient soil moisture for the plant to complete its life cycle. In such cases, only plants that have some water available for reproduction late in the season or those that complete their life cycle rapidly before the onset of drought (exhibiting drought escape) will produce seeds for the next generation. Both strategies allow for some reproductive success.
- The situation changes when summer rainfall is substantial but irregular. In this scenario, plants with large leaf areas or those capable of quickly developing such areas are better suited to take advantage of occasional wet summers. One acclimation strategy under these conditions is the ability to sustain both vegetative growth and flowering over an extended period. These plants are described as having an indeterminate growth habit, in contrast to determinate plants that produce a predetermined number of leaves and flower over a short period.
- In the subsequent discussions, we will explore various acclimation strategies, including inhibited leaf expansion, shedding of leaves, increased root growth, and closure of stomata.
Decreased Leaf Area Is an Early Adaptive Response to Water Deficit
- A reduced leaf area is one of the initial adaptive responses to water scarcity. Typically, as a plant experiences a decrease in water content, its cells shrink, and the cell walls become less rigid. This reduction in cell volume results in decreased turgor pressure and an increase in solute concentration within the cells. The plasma membrane becomes denser and more compact because it covers a smaller surface area than before.
- Because the reduction in turgor pressure is the earliest significant effect of water stress, activities dependent on turgor, such as leaf expansion and root elongation, are highly sensitive to water deficits (as depicted in Figure 25.1).

- Cell expansion is primarily driven by turgor pressure and is extremely sensitive to water deficit. The growth rate of a cell can be described using the formula GR = m(Yp – Y) (Equation 25.1), where GR represents the growth rate, Yp is turgor, Y is the yield threshold (the pressure below which the cell wall resists plastic, or non-reversible, deformation), and m is wall extensibility (the responsiveness of the wall to pressure).
- This equation demonstrates that a decrease in turgor leads to a reduction in growth rate. Additionally, it shows that to stop expansion, Yp only needs to decrease to the value of Y, not necessarily to zero. Under normal conditions, Y is typically only 0.1 to 0.2 MPa less than Yp, so even minor decreases in water content and turgor can slow down or entirely halt growth.
- Water stress not only reduces turgor but also decreases m and increases Y. Wall extensibility (m) is usually greatest when the cell wall solution is slightly acidic. Stress reduces m, partly due to an increase in cell wall pH during stress. The effects of stress on Y are not fully understood, but they likely involve complex structural changes in the cell wall (as discussed in Chapter 15) that may not be easily reversed after the relief of stress. Water-deficient plants often rehydrate at night, resulting in significant leaf growth during that time. Nevertheless, due to changes in m and Y, the growth rate remains lower than that of unstressed plants with the same turgor (as shown in Figure 25.1).
- Since leaf expansion primarily relies on cell expansion, the principles governing both processes are similar. Inhibiting cell expansion leads to an early reduction in leaf expansion in response to water deficits. A smaller leaf area transpires less water, effectively conserving the limited soil moisture over a longer period. Consequently, decreasing leaf area can be seen as an initial defense mechanism against drought.
- In plants with an indeterminate growth habit, water stress not only limits leaf size but also the number of leaves, as it reduces both the quantity and growth rate of branches. While less research has been conducted on stem growth compared to leaf expansion, it is likely affected by the same factors that limit leaf growth during stress.
- It's important to note that cell and leaf expansion also rely on biochemical and molecular factors beyond those associated with water flux. There is ample evidence indicating that plants adjust their growth rates in response to stress by coordinating various other essential processes, including cell wall and membrane biosynthesis, cell division, and protein synthesis (as mentioned by Burssens et al. in 2000).
Water Deficit Stimulates Leaf Abscission
- Water scarcity prompts adjustments in a plant's total leaf area, which is calculated as the product of the number of leaves and the surface area of each leaf. When plants experience water stress after a significant leaf area has developed, the leaves undergo senescence and eventually drop from the plant (as shown in Figure 25.2).

- This adaptive response is a crucial long-term change that enhances the plant's adaptability in a water-limited environment. In fact, many desert plants that shed all their leaves during drought conditions and sprout new ones after rain exemplify this strategy. This cycle can occur multiple times within a single season.
- Leaf abscission during water stress primarily results from an increased production of, and heightened sensitivity to, the plant hormone ethylene.
Water Deficit Enhances Root Extension into Deeper, Moist Soil
- Moderate water deficits also influence the development of the root system. The ratio of root biomass to shoot biomass seems to be regulated by the functional balance between water absorption by the roots and photosynthesis in the shoot. Essentially, a shoot continues to grow until it becomes so large that the roots' ability to absorb water becomes a limiting factor for further growth. Conversely, roots grow until their demand for energy from the shoot matches the supply. This balance is disrupted when water availability decreases.
- As mentioned earlier, leaf expansion is affected at an early stage when water uptake is restricted, whereas photosynthetic activity is less affected. Inhibiting leaf expansion reduces the consumption of carbon and energy, allowing a larger proportion of the plant's resources to be allocated to the root system, supporting additional root growth. Simultaneously, the root tips in dry soil lose turgor.
- These factors collectively promote the preferential growth of roots into the soil layers that retain moisture. As water deficits intensify, the uppermost soil layers typically dry out first. Consequently, plants often exhibit predominantly shallow root systems when all soil layers are adequately hydrated. However, as the upper soil layers become drier, there is a reduction in shallow roots and an increase in deep roots. The expansion of deeper roots into moist soil can be considered a secondary defense mechanism against drought.
- Increased root growth into moist soil zones during stress necessitates the allocation of assimilates (nutrient-rich compounds) to the growing root tips. During water scarcity, assimilates are redirected to the fruits and away from the roots. This redirection of resources results in less pronounced enhanced water uptake through root growth in reproductive plants compared to vegetative plants. The competition for assimilates between roots and fruits explains why plants are generally more sensitive to water stress during the reproductive phase.
Stomata Close during Water Deficit in Response to Abscisic Acid
- In situations where plants face rapid onset of stress or have already reached their maximum leaf area before the stress begins, they deploy different responses to protect themselves from immediate desiccation. Under such conditions, stomatal closure serves as a third defense mechanism against drought by reducing water loss through evaporation from the existing leaf area.
- Stomata, the tiny openings on the leaf surface, open and close in response to changes in the turgor (pressure) of specialized cells called guard cells. This turgor change controls the opening and closing of stomata. Since guard cells are located on the leaf epidermis, they can lose turgor due to direct water loss through evaporation into the atmosphere. This decrease in turgor leads to stomatal closure via a mechanism known as hydropassive closure. This mechanism is particularly relevant in low humidity conditions when the direct loss of water from guard cells to the atmosphere is too rapid for water to move into the guard cells from adjacent epidermal cells.
- Another mechanism, called hydroactive closure, involves metabolic processes within the guard cells and results in stomatal closure when either the entire leaf or the roots experience dehydration. This mechanism depends on changes in the solute content within the guard cells, causing water loss and a subsequent drop in turgor pressure, which leads to stomatal closure. Hydroactive closure essentially reverses the process of stomatal opening. However, the control of hydroactive closure differs subtly from that of stomatal opening.

- The loss of solutes from guard cells can be triggered by a decrease in leaf water content, and the plant hormone abscisic acid (ABA) plays a crucial role in this process. ABA is continuously synthesized at a low rate in mesophyll cells and tends to accumulate in chloroplasts. When mesophyll cells become mildly dehydrated, two key events occur:
- Some of the ABA stored in chloroplasts is released into the apoplast (the space within the cell wall) of mesophyll cells. The redistribution of ABA relies on pH gradients within the leaf, the weak-acid properties of the ABA molecule, and membrane permeability properties. This redistribution enables some ABA to be transported to the guard cells through the transpiration stream.
- ABA is synthesized at a higher rate, leading to increased ABA accumulation in the leaf apoplast. Elevated ABA concentrations resulting from increased synthesis appear to reinforce or prolong the initial closure effect of stored ABA.
- Stomatal responses to leaf dehydration can vary widely, both within and between plant species. Some dehydration-delaying species, such as cowpea and cassava, exhibit particularly responsive stomata that decrease stomatal conductance and transpiration to the extent that leaf water potential remains nearly constant during drought.
- Chemical signals from the root system can influence stomatal responses to water stress. Stomatal conductance often correlates more closely with soil water status than leaf water status. The root system is the only plant part that can directly sense soil water status, and even dehydrating a portion of the root system may lead to stomatal closure, even if the well-watered portion of the roots can still supply sufficient water to the shoots.
- For example, when corn plants were grown with roots extending into two separate pots and water was withheld from one of the pots, the stomata partially closed, and leaf water potential increased, similar to the dehydration-delaying plants mentioned earlier. These findings indicate that stomata can respond to conditions sensed in the roots.
- Apart from ABA, other signals such as pH and redistribution of inorganic ions are believed to play a role in long-distance communication between roots and shoots.
Water Deficit Limits Photosynthesis within the Chloroplast
- The impact of mild water stress on the photosynthetic rate of leaves (expressed per unit leaf area) is typically not as pronounced as its effect on leaf expansion (as shown in Figure 25.4). This is because photosynthesis is much less sensitive to changes in turgor pressure compared to leaf expansion. Nonetheless, mild water stress generally affects both leaf photosynthesis and stomatal conductance. As stomata close during the early stages of water stress, there may be an increase in water-use efficiency, meaning that more carbon dioxide (CO2) is taken up per unit of transpired water. This is because stomatal closure reduces transpiration more than it decreases the concentration of CO2 inside the leaf.

- However, as stress becomes more severe, the dehydration of mesophyll cells begins to inhibit photosynthesis, and mesophyll metabolism is impaired. Under such conditions, water-use efficiency typically decreases. Numerous studies have consistently shown that the relative impact of water stress on stomatal conductance is significantly greater than its effect on photosynthesis. To assess the direct influence of water stress on photosynthesis, experiments expose stressed leaves to air containing high concentrations of CO2. This eliminates any impact of stress on stomatal conductance, allowing the differences in photosynthetic rates between stressed and unstressed plants to be directly attributed to damage to photosynthesis caused by water stress.
- Does water stress have a direct effect on translocation, the movement of assimilates (sugars and other nutrients) within the plant? Water stress does reduce both photosynthesis and the utilization of assimilates in expanding leaves. Consequently, it indirectly reduces the amount of photosynthate exported from the leaves. Since the movement of assimilates in the phloem (the plant's vascular tissue responsible for nutrient transport) relies on turgor pressure (as explained in Chapter 10), the decreased water potential in the phloem during stress may inhibit assimilate movement. However, experiments have shown that translocation remains unaffected until late in the stress period, when other processes such as photosynthesis have already been significantly inhibited (as shown in Figure 25.5).
 FIGURE 25.5: Relative effects of water stress on photosyn- thesis and translocation in sorghum (Sorghum bicolor). Plants were exposed to 14CO2 for a short time interval. The radioactivity fixed in the leaf was taken as a measure of photosynthesis, and the loss of radioactivity after removal of the 14CO2 source was taken as a measure of the rate of assimilate translocation. Photosynthesis was affected by mild stress, whereas, translocation was unaffected until stress was severe. (After Sung and Krieg 1979.)
FIGURE 25.5: Relative effects of water stress on photosyn- thesis and translocation in sorghum (Sorghum bicolor). Plants were exposed to 14CO2 for a short time interval. The radioactivity fixed in the leaf was taken as a measure of photosynthesis, and the loss of radioactivity after removal of the 14CO2 source was taken as a measure of the rate of assimilate translocation. Photosynthesis was affected by mild stress, whereas, translocation was unaffected until stress was severe. (After Sung and Krieg 1979.)
- This relative insensitivity of translocation to water stress enables plants to mobilize and use reserves where they are most needed, such as during seed growth, even when stress is severe. The ability to continue transporting assimilates is a crucial factor in nearly all aspects of a plant's resistance to drought.
Osmotic Adjustment of Cells Helps Maintain Plant Water Balance
- Osmotic adjustment is a process employed by plants to help them maintain water balance as the soil becomes drier, and the matric potential of the soil becomes more negative. For plants to continue absorbing water, their water potential (Yw) must be lower (more negative) than that of the soil water. Osmotic adjustment involves the accumulation of solutes within plant cells, which results in a decrease in water potential without a concurrent decrease in turgor or cell volume. The change in cell water potential is mainly due to changes in solute potential (Ys), which constitutes the osmotic component of Yw.
- Osmotic adjustment essentially refers to a net increase in solute content per cell that is independent of any changes in cell volume resulting from water loss. Typically, the reduction in Ys ranges from approximately 0.2 to 0.8 MPa, except in plants adapted to extremely arid conditions. This adjustment is largely accounted for by an increase in the concentration of various common solutes, including sugars, organic acids, amino acids, and inorganic ions (particularly K+).
- High concentrations of ions can severely inhibit the cytosolic enzymes in plant cells. During osmotic adjustment, ions are primarily accumulated within vacuoles, where they are isolated from contact with enzymes in the cytoplasm or subcellular organelles. To maintain water potential equilibrium within the cell, other solutes, referred to as compatible solutes or compatible osmolytes, accumulate in the cytoplasm. These compatible solutes are organic compounds that do not interfere with enzyme functions and include substances like the amino acid proline, sugar alcohols such as sorbitol and mannitol, and a quaternary amine called glycine betaine. The synthesis of compatible solutes helps plants adapt to increased salinity in the root zone, as discussed later in this chapter.
- Osmotic adjustment occurs gradually in response to tissue dehydration, taking place over several days while other changes, such as growth or photosynthesis, are also occurring. Therefore, it can be argued that osmotic adjustment is not an independent and direct response to water deficit but rather a result of other factors, such as a reduced growth rate. Nevertheless, leaves capable of osmotic adjustment can clearly maintain turgor at lower water potentials than those that cannot adjust. This ability to sustain turgor enables continued cell elongation and allows for higher stomatal conductance even at lower water potentials. This suggests that osmotic adjustment is an adaptive response that enhances drought tolerance.
- But how much additional water can a plant acquire through osmotic adjustment in leaf cells? Most of the soil's extractable water is found in spaces that are easily accessible to roots (as explained in Chapter 4). As the soil dries, this readily available water is utilized first, leaving behind a smaller amount of water held more tightly in small pores. Osmotic adjustment enables the plant to extract a bit more of this tightly held water. However, the increase in total available water is relatively small. Therefore, the benefits of osmotic adjustment in leaves need to be weighed against the diminishing returns in terms of water availability to the plant. Comparing the water relations of adjusting and nonadjusting species (as shown in Figure 25.6), it becomes evident that osmotic adjustment promotes tolerance to dehydration but has a limited impact on overall productivity.

FIGURE 25.6: Water loss and carbon gain by sugar beet (Beta vulgaris), an osmotically adjusting species, and cowpea (Vigna unguiculata), a nonadjusting species that conserves water during stress by stomatal closure. Plants were grown in pots and subjected to water stress. On any given day after the last watering, the sugar beet leaves maintained a lower water potential than the cowpea leaves, but photo- synthesis and transpiration during stress were only slightly greater in the sugar beet. The major difference between the two plants was the leaf water potential. These results show that osmotic adjustment promotes dehydration tolerance but does not have a major effect on productivity. (After McCree and Richardson 1987.)
- Osmotic adjustment can also occur in plant roots, although this process has not been as extensively studied as in leaves. While the absolute magnitude of osmotic adjustment in roots is typically less than in leaves, it can represent a larger percentage of the original tissue solute potential (Ys). As with leaves, these changes may only slightly increase water extraction from previously explored soil. However, osmotic adjustment can occur in root meristems, enhancing turgor pressure and maintaining root growth. This is a vital aspect of the alterations in root growth patterns as water becomes scarcer in the soil.
- The question of whether osmotic adjustment can boost plant productivity has been examined by researchers. They have attempted to enhance the accumulation of osmoprotective solutes through traditional plant breeding, physiological methods (inducing adjustment with controlled water deficits), and the use of transgenic plants expressing genes for solute synthesis and accumulation. However, engineered plants have exhibited slower growth and only slightly improved tolerance to osmotic stresses. Consequently, the practical utilization of osmotic adjustment to enhance agricultural performance remains a challenge yet to be fully realized.
Water Deficit Increases Wax Deposition on the Leaf Surface
- When plants experience water stress, they commonly respond by developing a thicker cuticle, which serves to reduce water loss from the epidermis through a process known as cuticular transpiration. This response involves the deposition of waxes, both on the leaf surface and within the inner layer of the cuticle. It's worth noting that while wax accumulation occurs in response to water deficit, the inner layer of the cuticle may play a more critical role in regulating water loss, and this regulation is more complex than simply increasing the quantity of wax present.
- The thicker cuticle also has the effect of reducing the permeability of carbon dioxide (CO2). However, leaf photosynthesis remains unaffected by this change because the epidermal cells beneath the cuticle are non-photosynthetic and don't participate in the photosynthetic process. It's important to emphasize that cuticular transpiration typically accounts for only a small portion of total leaf transpiration, usually ranging from 5% to 10%. Therefore, it becomes a significant factor in water loss only under extremely severe stress conditions or when the cuticle itself has been damaged, for instance, by wind-driven sand or other external factors.
Water Deficit Alters Energy Dissipation from Leaves
- Water deficit can have a significant impact on the way energy is dissipated from leaves. Evaporative heat loss during transpiration serves to cool leaves. This cooling effect can be quite substantial. For example, in extremely hot regions like Death Valley, California, where temperatures soar, plants with access to ample water can maintain leaf temperatures as much as 8°C below the surrounding air temperature. In warm and arid climates, experienced farmers can often gauge a plant's need for water simply by touching the leaves, as a leaf undergoing rapid transpiration feels noticeably cooler to the touch.
- The interrelationship between water stress and heat stress is evident in these effects of transpiration on leaf temperature. To sustain a leaf temperature significantly lower than the air temperature, a large quantity of water must be evaporated. This is why adaptations that cool leaves through means other than evaporation, such as changes in leaf size and orientation, are highly effective in conserving water. When transpiration decreases, and leaf temperature begins to rise above the air temperature, some of the excess energy within the leaf is dissipated as sensible heat loss.
- Many plants adapted to arid zones have small leaves, which helps minimize the resistance of the boundary layer for the transfer of heat from the leaf to the air. Consequently, small leaves tend to remain close to the air temperature even when transpiration rates decrease significantly. In contrast, larger leaves have higher boundary layer resistance and dissipate less thermal energy per unit leaf area through direct heat transfer to the air.
- In addition to leaf size, leaf movement can play a role in protecting leaves from overheating during water stress. Some leaves have the ability to orient themselves away from direct sunlight, known as paraheliotropic movement, while others gain energy by positioning themselves perpendicular to the sunlight, referred to as diaheliotropic movement. Water stress can influence leaf positioning, and other factors like wilting or leaf rolling in grasses can also alter the leaf's exposure to sunlight.
- Moreover, the absorption of energy can be reduced through various adaptations. Some plants have densely packed hairs on the leaf surface or layers of reflective wax outside the cuticle, giving the leaves a gray-white appearance. These features reflect a substantial amount of light, helping to keep the leaves cooler by reducing radiation absorption. However, such pubescence can also decrease the absorption of visible wavelengths crucial for photosynthesis, thus impacting carbon assimilation. This trade-off has limited the success of attempts to breed pubescent traits into crops as a means to improve water-use efficiency.
Osmotic Stress Induces Crassulacean Acid Metabolism in Some Plants
- Crassulacean acid metabolism (CAM) is a unique plant adaptation characterized by the opening of stomata at night and their closure during the day. This strategy takes advantage of the reduced vapor pressure difference between the leaf and the surrounding air at night when both leaf and air temperatures are lower. As a result, CAM plants exhibit exceptionally high water-use efficiency, with the ability to gain 1 gram of dry matter for every 125 grams of water used. This ratio is three to five times greater than that observed in typical C3 plants.
- CAM is particularly prevalent in succulent plants, including cacti. Some succulent species can exhibit facultative CAM, meaning they switch to CAM metabolism in response to water deficits or saline conditions. This metabolic switch is a remarkable adaptation to stress and involves the accumulation of specific enzymes such as phosphoenolpyruvate (PEP) carboxylase, pyruvate–orthophosphate dikinase, and NADP malic enzyme, among others.
- CAM metabolism encompasses various structural, physiological, and biochemical features. These include alterations in carboxylation and decarboxylation patterns, the transport of significant amounts of malate into and out of vacuoles, and a reversal in the periodicity of stomatal movements. Consequently, CAM induction represents an extraordinary adaptation to water deficit stress that operates at multiple levels of plant organization.

 FIGURE 25.7: Orientation of leaflets of field-grown soybean (Glycine max) plants in the normal, unstressed, position (A); during mild water stress (B); and during severe water stress (C). The large leaf movements induced by mild stress are quite different from wilting, which occurs during severe stress. Note that during mild stress (B), the terminal leaflet has been raised, whereas the two lateral leaflets have been lowered; each is almost vertical. (Courtesy of D. M. Oosterhuis.)
FIGURE 25.7: Orientation of leaflets of field-grown soybean (Glycine max) plants in the normal, unstressed, position (A); during mild water stress (B); and during severe water stress (C). The large leaf movements induced by mild stress are quite different from wilting, which occurs during severe stress. Note that during mild stress (B), the terminal leaflet has been raised, whereas the two lateral leaflets have been lowered; each is almost vertical. (Courtesy of D. M. Oosterhuis.)
Osmotic Stress Changes Gene Expression
Osmotic stress induces significant changes in gene expression as plants respond to the need for osmotic adjustment and adapt to stressful conditions. These alterations in gene expression are crucial for the synthesis and accumulation of compatible solutes and other compounds that help the plant cope with osmotic stress.
Some key points related to osmotic stress-induced gene expression include:
- Up-regulated Genes: Osmotic stress leads to the up-regulation (increased expression) of several genes associated with osmotic adjustment. These genes encode enzymes involved in the biosynthesis of compatible solutes, such as proline, glycine betaine, and pinitol. For example, genes coding for ∆′1-Pyrroline-5-carboxylate synthase (proline biosynthesis), Betaine aldehyde dehydrogenase (glycine betaine accumulation), and myo-Inositol 6-O-methyltransferase (pinitol synthesis) are activated under osmotic stress.
- Down-regulated Genes: In contrast, some genes involved in mannitol biosynthesis are not up-regulated during osmotic stress. Instead, osmotic stress may down-regulate genes responsible for sucrose production and mannitol degradation, leading to enhanced mannitol accumulation as a protective response.

- Membrane Transport: Osmotic stress also influences the expression of genes associated with membrane transport, including ATPases and aquaporins (water channel proteins). These genes play roles in ion transport and water movement, helping the plant adapt to changing osmotic conditions.
- Proteases and Heat Shock Proteins: Certain protease genes are induced by osmotic stress, potentially facilitating the degradation of denatured proteins caused by stress. Heat shock proteins are also osmotically induced and may assist in protecting or renaturing proteins affected by desiccation.

- Cell Wall-Related Genes: Osmotic stress affects genes involved in maintaining the structural composition and integrity of cell walls. Genes coding for enzymes like S-adenosylmethionine synthase and peroxidases, which are implicated in lignin biosynthesis, can be controlled by stress.
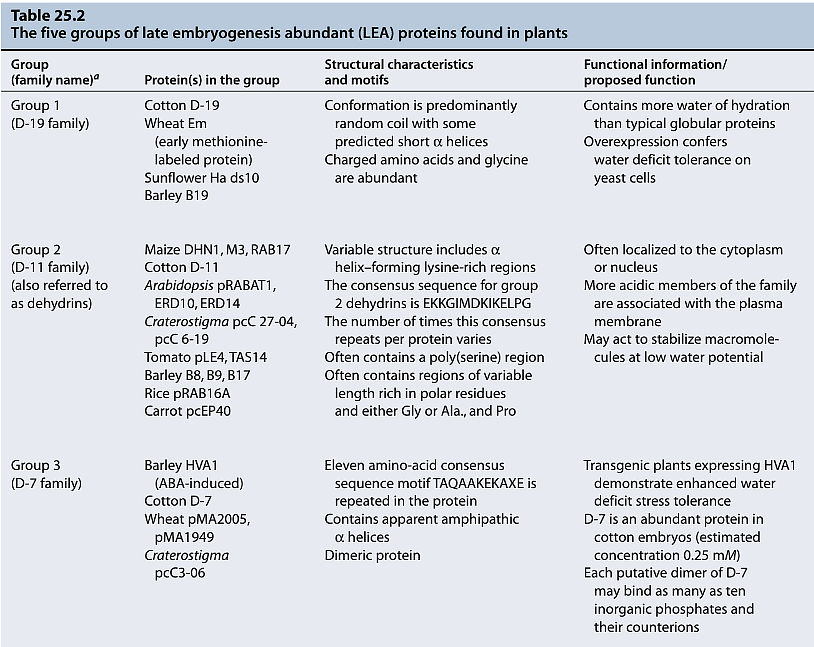
- LEA Proteins: Late embryogenesis abundant (LEA) proteins, which accumulate during desiccation, are thought to have a role in protecting cellular membranes during osmotic stress. They are hydrophilic and capable of binding water, which might help prevent the crystallization of vital cellular components and contribute to membrane stabilization.
- Genome-Wide Expression: Microarray techniques have been employed to analyze the entire genomes of plants in response to osmotic stress. These studies have revealed that numerous genes exhibit changes in expression levels when plants are exposed to osmotic stress, underscoring the complexity of the stress response at the genetic level.
- ABA-Responsive Genes: Osmotic stress typically results in the accumulation of abscisic acid (ABA), an important stress hormone. As a consequence, products of ABA-responsive genes also accumulate during osmotic stress. However, not all osmotic stress-induced genes are regulated by ABA, suggesting the involvement of other regulatory mechanisms.
Overall, osmotic stress-induced changes in gene expression are a fundamental part of a plant's response to challenging environmental conditions, enabling it to adapt and enhance its tolerance to osmotic stress.
Stress-Responsive Genes Are Regulated by ABA-Dependent and ABA-Independent Processes
Gene expression in response to stress, such as osmotic stress, is tightly regulated by complex signaling pathways involving various transcription factors and regulatory sequences in gene promoters.
Here are some key points regarding the regulation of stress-responsive genes by both ABA-dependent and ABA-independent processes:
- Transcription Factor Interaction: Gene transcription is controlled through the interaction of transcription factors with specific regulatory sequences in gene promoters. These transcription factors are proteins that bind to these regulatory sequences, thereby influencing gene expression.
- Specific Regulatory Sequences: Different genes induced by the same stress signal, like osmotic stress, have specific regulatory sequences in their promoters. For example, the RD29 gene can be activated by osmotic stress, cold, and ABA, and its promoter contains specific DNA sequences for these responses.
- ABA-Regulated Genes: Genes that are regulated by ABA contain a six-nucleotide sequence element known as the ABA response element (ABRE) in their promoters. ABRE is recognized by transcription factors involved in ABA-regulated gene activation.
- ABA-Dependent and ABA-Independent Genes: Genes responsive to osmotic stress can be categorized into two main groups: ABA-dependent genes and ABA-independent genes. ABA-dependent genes are regulated through ABA-mediated signaling pathways, while ABA-independent genes are controlled by separate osmotic stress-responsive signaling pathways.
- DREB Transcription Factors: ABA-independent, osmotic stress-responsive genes are regulated by transcription factors known as DREB1 and DREB2. These factors bind to specific DRE (dehydration response element) sequences in gene promoters and are activated by signaling cascades independent of ABA.
- MAP Kinase Signaling: Some ABA-independent, osmotic stress-responsive genes are directly controlled by the MAP kinase signaling cascade, which involves protein kinases. This cascade transmits signals that lead to changes in gene expression.
- Complexity and Cross-Talk: The regulation of gene expression during osmotic stress involves a complex network of interactions, including cross-talk between different signaling pathways. This complexity reflects the intricate relationship between gene expression and physiological adaptations to osmotic stress, allowing plants to respond effectively to changing environmental conditions.

Overall, the ability of plants to respond to osmotic stress involves multiple layers of regulation, with both ABA-dependent and ABA-independent pathways playing important roles in coordinating stress responses at the genetic level.
Heat Stress and Heat Shock
- Heat stress is a significant challenge for most plant tissues, as they are generally unable to survive prolonged exposure to temperatures exceeding 45°C. However, non-growing cells or dehydrated tissues, such as seeds and pollen, can endure higher temperatures than hydrated, actively growing cells. For instance, dry seeds can withstand temperatures of up to 120°C, while some pollen grains can survive temperatures as high as 70°C. Actively growing plant tissues typically do not survive temperatures above 45°C, making them highly vulnerable to heat stress.
- Interestingly, brief exposures to sublethal heat stresses can induce a level of tolerance to otherwise lethal temperatures, a phenomenon known as induced thermotolerance. We'll explore the mechanisms underlying induced thermotolerance later in this chapter.

- It's worth noting that the relationship between water stress and heat stress is interconnected. In many cases, well-hydrated plant shoots can stay below 45°C due to evaporative cooling. However, if water availability becomes limited, evaporative cooling diminishes, leading to higher tissue temperatures. One exception to this rule is emerging seedlings in moist soil, which can face greater heat stress compared to those in drier soils. This is because wet, bare soil tends to be darker and absorbs more solar radiation than drier soil, contributing to higher temperatures in these conditions.
High Leaf Temperature and Water Deficit Lead to Heat Stress
- Many CAM plants, which are succulent and adapted to high temperatures, have the ability to tolerate tissue temperatures ranging from 60 to 65°C, even in conditions of intense solar radiation during summer. This adaptation is essential for CAM plants as they keep their stomata closed during the day, preventing them from cooling through transpiration. Instead, they manage high temperatures by dissipating heat from incoming solar radiation through methods like re-emitting long-wave (infrared) radiation and heat loss through conduction and convection.
- In contrast, typical non-irrigated C3 and C4 plants rely on transpirational cooling to maintain lower leaf temperatures. However, under certain conditions, such as soil water deficits or high relative humidity that reduces evaporative cooling potential, the leaf temperature can increase by 4 to 5°C above the surrounding air temperature, especially during bright sunlight near midday. The physiological impacts of such temperature increases are discussed in the following section.
- Significant increases in leaf temperature can occur in plants from arid and semi-arid regions, particularly when they experience drought and strong sunshine. Heat stress can also be a concern in greenhouse environments where low air movement and high humidity reduce leaf cooling rates. Moderate heat stress can inhibit overall plant growth. Some irrigated crops, like cotton, rely on transpirational cooling to dissipate heat, and this enhanced cooling is associated with higher agricultural yields in irrigated cotton production.
At High Temperatures, Photosynthesis Is Inhibited before Respiration
- Both photosynthesis and respiration experience inhibition at high temperatures, but photosynthetic rates decrease before respiratory rates do as temperature rises (Figure 25.10A and B). The temperature at which the amount of CO2 fixed by photosynthesis equals the amount of CO2 released by respiration over a given time period is known as the temperature compensation point.
- At temperatures exceeding the temperature compensation point, photosynthesis cannot replenish the carbon used as a substrate for respiration. Consequently, carbohydrate reserves decrease, and the sweetness of fruits and vegetables diminishes. This imbalance between photosynthesis and respiration is a major factor contributing to the adverse effects of high temperatures.
- Within the same plant, the temperature compensation point is generally lower for shade leaves compared to sun leaves that receive direct exposure to light and heat. Elevated respiration rates relative to photosynthesis at high temperatures have more detrimental effects on C3 plants than on C4 or CAM plants because higher temperatures increase the rates of both dark respiration and photorespiration in C3 plants.
 FIGURE 25.10: Response of frosted orache (Atriplex sabulosa) and Arizona honeysweet (Tidestromia oblongifolia) to heat stress. Photosynthesis (A) and respiration (B) were measured on attached leaves, and ion leakage (C) was measured in leaf slices submerged in water. At the beginning of the experiment, control rates were measured at a noninjurious 30°C. Attached leaves were then exposed to the indicated temperatures for 15 minutes and returned to the initial control conditions before the rates were recorded. Arrows indicate the temperature thresholds for inhibi- tion of photosynthesis in each of the two species. Photosynthesis, respiration, and membrane permeability were all more sensitive to heat damage in A. sabulosa than in T. oblongifolia. In both species, however, photosynthesis was more sensitive to heat stress than either of the other two processes, and photosynthesis was completely inhibited at temperatures that were noninjurious to respiration. (From Björkman et al. 1980.)
FIGURE 25.10: Response of frosted orache (Atriplex sabulosa) and Arizona honeysweet (Tidestromia oblongifolia) to heat stress. Photosynthesis (A) and respiration (B) were measured on attached leaves, and ion leakage (C) was measured in leaf slices submerged in water. At the beginning of the experiment, control rates were measured at a noninjurious 30°C. Attached leaves were then exposed to the indicated temperatures for 15 minutes and returned to the initial control conditions before the rates were recorded. Arrows indicate the temperature thresholds for inhibi- tion of photosynthesis in each of the two species. Photosynthesis, respiration, and membrane permeability were all more sensitive to heat damage in A. sabulosa than in T. oblongifolia. In both species, however, photosynthesis was more sensitive to heat stress than either of the other two processes, and photosynthesis was completely inhibited at temperatures that were noninjurious to respiration. (From Björkman et al. 1980.)
Plants Adapted to Cool Temperatures Acclimate Poorly to High Temperatures
- The ability of plants adapted to a specific temperature range to adjust to significantly different temperature conditions can be demonstrated by comparing the responses of two C4 plant species: Atriplex sabulosa (frosted orache) and Tidestromia oblongifolia (Arizona honeysweet).
- Atriplex sabulosa is naturally found in the cooler climate of coastal northern California, while Tidestromia oblongifolia thrives in the extremely hot environment of Death Valley, California, which is often fatal to most plant species. When these two species were cultivated in a controlled environment, their growth rates were monitored at various temperatures.
- T. oblongifolia displayed minimal growth at 16°C, whereas A. sabulosa still achieved 75% of its maximum growth rate at this temperature. However, as temperatures increased, A. sabulosa's growth rate began to decline between 25 and 30°C, eventually ceasing at 45°C. In contrast, T. oblongifolia exhibited its highest growth rate at 45°C (see Figure 25.10A). These results clearly indicate that neither species could adapt to the temperature range of the other.
High Temperature Reduces Membrane Stability
- The stability of cellular membranes is crucial during periods of high-temperature stress, much like it is during cold exposure and freezing. Excessive fluidity of membrane lipids at elevated temperatures is associated with a loss of normal physiological functions. In plants like oleander (Nerium oleander), adaptation to high temperatures involves an increase in the saturation level of fatty acids in membrane lipids, making the membranes less fluid (Raison et al. 1982).
- At elevated temperatures, the strength of hydrogen bonds and electrostatic interactions between polar groups of proteins within the aqueous phase of the membrane decreases. These high temperatures lead to modifications in membrane composition and structure, potentially causing the leakage of ions (see Figure 25.10C). Such membrane disruption can also result in the inhibition of processes like photosynthesis and respiration, which rely on the activity of membrane-associated electron carriers and enzymes.
- Photosynthesis is particularly sensitive to high temperatures (see Chapter 9). In a study involving Atriplex and Tidestromia, conducted by O. Björkman and colleagues in 1980, it was found that electron transport in photosystem II was more susceptible to high temperatures in the cold-adapted A. sabulosa than in the heat-adapted T. oblongifolia. In both plant species, enzymes such as ribulose-1,5-bisphosphate carboxylase, NADP:glyceraldehyde-3-phosphate dehydrogenase, and phosphoenolpyruvate carboxylase exhibited reduced stability at high temperatures in A. sabulosa compared to T. oblongifolia.
- However, it's noteworthy that the temperatures at which these enzymes began to denature and lose activity were significantly higher than the temperatures at which photosynthesis started to decline. These findings suggest that the initial stages of heat-induced damage to photosynthesis are more directly linked to alterations in membrane properties and the disruption of energy transfer mechanisms within chloroplasts, rather than a general denaturation of proteins.
Several Adaptations Protect Leaves against Excessive Heating
- In environments characterized by intense solar radiation and high temperatures, plants employ strategies to prevent excessive heating of their leaves. This adaptation is particularly crucial in warm, sunny habitats where a transpiring leaf is close to its upper temperature tolerance limit. In such conditions, any additional warming due to reduced water evaporation or increased energy absorption can be detrimental to the leaf.
- Plants use a range of adaptations to achieve both drought resistance and heat resistance, including:
- Reflective leaf hairs and leaf waxes: Some plants develop leaf structures that reflect sunlight, reducing the amount of solar radiation absorbed by the leaves. Leaf hairs and waxes on the leaf surface can help in this regard.
- Leaf rolling and vertical leaf orientation: Certain plants have leaves that can roll up or orient vertically to reduce their exposure to direct sunlight. This minimizes the absorption of solar energy and helps keep leaf temperatures in check.
- Growth of small, highly dissected leaves: Some plants produce small, deeply divided leaves. This morphology serves to minimize the thickness of the boundary layer around the leaves, maximizing the transfer of heat through convection and conduction, which aids in dissipating excess heat.
- Dimorphic leaves: Certain desert shrubs, like white brittlebush (Encelia farinosa, family Compositae), exhibit two types of leaves to prevent excessive heating. During the winter, these plants have green, nearly hairless leaves, while in the summer, they produce white, pubescent (hairy) leaves. This change in leaf characteristics helps regulate temperature and minimize heat stress.
- These adaptations collectively enable plants to cope with high temperatures and intense solar radiation by reducing heat absorption and facilitating heat dissipation, thereby protecting their leaves from damage caused by excessive heating.
At Higher Temperatures, Plants Produce Heat Shock Proteins
- When plants experience a sudden temperature increase of 5 to 10°C, they respond by producing a specialized group of proteins known as heat shock proteins (HSPs). These HSPs play a crucial role in helping cells withstand heat stress by acting as molecular chaperones. Heat stress can cause proteins within the cell, which serve as enzymes or structural components, to lose their proper shape and function as they become unfolded or misfolded. This misfolding often leads to the aggregation and precipitation of these proteins, disrupting normal cellular processes. HSPs act as molecular chaperones by facilitating the correct folding of misfolded or aggregated proteins and preventing further misfolding, thereby ensuring proper cellular functioning even under elevated, stressful temperatures.
- Heat shock proteins were initially discovered in fruit flies (Drosophila melanogaster) and have since been found in various organisms, including animals, humans, plants, fungi, and microorganisms. For instance, when soybean seedlings are exposed to a sudden temperature shift from 25 to 40°C (just below the lethal temperature range), the synthesis of typical cellular mRNAs and proteins is suppressed, while the transcription and translation of a distinct set of 30 to 50 proteins (HSPs) are enhanced. New mRNA transcripts for HSPs can be detected within minutes of heat shock.
- It's worth noting that while plant HSPs were initially identified in response to abrupt temperature changes (25 to 40°C), which are rare in nature, they can also be induced by more gradual temperature increases typical of the natural environment. Some HSPs are naturally present in unstressed cells, and certain essential cellular proteins share similarities with HSPs but do not increase in response to thermal stress.
- Heat shock proteins in plants come in various sizes, ranging from 15 to 104 kilodaltons (kDa), and they can be categorized into five classes based on size. Different HSPs are found in specific cellular compartments, including the nucleus, mitochondria, chloroplasts, endoplasmic reticulum, and cytosol. Members of certain HSP groups, such as HSP60, HSP70, HSP90, and HSP100, act as molecular chaperones by assisting in the ATP-dependent stabilization and folding of proteins and the assembly of oligomeric proteins. Some HSPs also aid in the transport of polypeptides across cellular membranes into various compartments. While HSP90s are associated with hormone receptors in animal cells, their role in plant cells remains less understood.
- In plants, low-molecular-weight HSPs (15–30 kDa) are more abundant than in other organisms. Plants have multiple classes of these small HSPs, found in the cytosol, chloroplasts, endoplasmic reticulum, and mitochondria, although their precise functions are not yet fully understood.
- Cells that synthesize HSPs in response to heat stress exhibit improved thermal tolerance and can endure temperatures that would otherwise be lethal. Notably, some HSPs are not exclusively induced by high-temperature stress; they can also be triggered by various environmental stressors, including water deficit, abscisic acid (ABA) treatment, physical damage (wounding), low temperatures, and salinity. As a result, cells that have previously encountered one form of stress may develop cross-protection against other stressors. For example, exposing tomato fruits to a heat shock treatment has been observed to promote the accumulation of HSPs and protect the cells from chilling stress at low temperatures for an extended period.

A Transcription Factor Mediates HSP Accumulation in Response to Heat Shock
- In all cells, there are molecular chaperones known as heat shock cognate proteins that are consistently expressed and function similarly to heat shock proteins (HSPs). However, when cells encounter a stressful but non-lethal heat episode, the production of HSPs significantly increases, while the synthesis of other proteins is greatly reduced or halted. This response to heat stress appears to be orchestrated by a specific transcription factor called heat shock factor (HSF), which plays a role in the transcription of HSP mRNA.
- Under normal conditions without heat stress, HSF exists as individual units (monomers) that cannot bind to DNA and initiate transcription (as shown in Figure 25.11). However, when cells experience stress, HSF monomers come together to form trimers, enabling them to bind to specific DNA sequences known as heat shock elements (HSEs). Once bound to the HSE, the trimeric HSF is modified through a process called phosphorylation, and this modification facilitates the transcription of HSP mRNA. Subsequently, HSP70 binds to HSF, leading to the separation of the HSF/HSE complex. The HSF is then returned to its monomeric form, ready for further cycles. In this way, through the action of HSF, HSPs accumulate until their levels are sufficient to interact with HSF, causing a halt.

HSPs Mediate Thermotolerance
- The conditions that trigger the development of thermal tolerance in plants align closely with those that stimulate the accumulation of heat shock proteins (HSPs). However, simply observing this correlation does not conclusively establish the essential role of HSPs in adapting to heat stress. More definitive experiments have demonstrated that the activation of heat shock factor (HSF) induces the continuous production of HSPs and enhances the thermotolerance of Arabidopsis plants. Studies involving Arabidopsis plants modified with an antisense DNA sequence to reduce HSP70 synthesis revealed that these mutant plants could survive at temperatures 2°C lower than the controls, although they grew normally at optimal temperatures (as reported by Lee and Schoeffl in 1996).
- It is presumed that the failure to produce the entire spectrum of HSPs typically induced in plants would lead to a more significant loss of thermotolerance. Other research involving Arabidopsis mutants (as conducted by Hong and Vierling in 2000) and genetically modified plants (as demonstrated by Queitsch et al. in 2000) has provided evidence that HSP101 is a crucial component of both induced and constitutive thermotolerance in plants.
Adaptation to Heat Stress Is Mediated by Cytosolic Calcium
- Enzymes that participate in various metabolic pathways may exhibit different responses to temperature, and this differential thermostability can impact specific metabolic steps before HSPs can restore their activity through their molecular chaperone function. Consequently, heat stress can induce changes in metabolism, resulting in the accumulation of certain metabolites and the reduction of others. These alterations can significantly disrupt the functioning of metabolic pathways, leading to imbalances that are challenging to rectify.
- Furthermore, heat stress can influence the rate of metabolic reactions that either consume or produce protons. It can also affect the activity of proton-pumping ATPases, which transport protons from the cytosol into the apoplast or vacuoles (refer to Chapter 6 for more information). This alteration might lead to cytosolic acidification, potentially causing additional disturbances in metabolism during stress. Cells possess metabolic acclimation mechanisms to mitigate these effects of heat stress on metabolism.
- One such metabolic acclimation response to heat stress involves the accumulation of the nonprotein amino acid γ-aminobutyric acid (GABA). Under heat stress conditions, GABA levels can increase six- to tenfold compared to unstressed plants. GABA is synthesized from the amino acid L-glutamate through a single reaction catalyzed by the enzyme glutamate decarboxylase (GAD). GAD is one of several enzymes regulated by the calcium-activated protein calmodulin, which acts as a regulatory protein. Calcium-activated calmodulin activates GAD, leading to an elevated rate of GABA biosynthesis (as depicted in Figure 25.12). Studies involving transgenic plants expressing the calcium-sensing protein aequorin have demonstrated that high-temperature stress results in increased cytosolic calcium levels, and these elevated calcium levels trigger the activation of GAD through calmodulin. Consequently, this leads to the accumulation of GABA induced by high temperatures.
- While GABA serves as an essential signaling molecule in the brain tissue of mammals, there is currently no evidence suggesting a similar role in plants. The precise functions of GABA in heat stress resistance are still being explored.

Chilling and Freezing
- Chilling temperatures are those that are too cold for normal plant growth but not cold enough to freeze water into ice. Typically, plants from tropical and subtropical regions are susceptible to chilling injury. This sensitivity to cold extends to various crops like maize, Phaseolus beans, rice, tomatoes, cucumbers, sweet potatoes, and cotton. Chilling-sensitive ornamental plants include Passiflora, Coleus, and Gloxinia.
- When plants accustomed to relatively warm temperatures (between 25 to 35°C) are suddenly exposed to cooler temperatures ranging from 10 to 15°C, they experience chilling injury. Symptoms of chilling injury include slowed growth, discoloration or lesions on leaves, and foliage appearing soggy, as if it has been soaked in water for an extended period.
- Chilling can also lead to wilting if the roots are affected. However, the response to chilling temperatures can vary even among species generally considered sensitive to cold. Some plants can develop improved resistance to chilling through genetic adaptations to colder conditions at high altitudes (as depicted in Figure 25.13). Furthermore, their resistance often increases when plants are first acclimated to cool but noninjurious temperatures. Gradual exposure to lower temperatures is more likely to minimize chilling damage, whereas abrupt exposure to near-freezing temperatures, known as cold shock, significantly raises the risk of injury.

- On the other hand, freezing injury occurs at temperatures below the freezing point of water. Similar to chilling, full development of freezing tolerance requires a period of acclimation to cold temperatures.
- In the subsequent discussion, we will explore how chilling injury affects membrane properties, the damage caused by ice crystals to cells and tissues, and the roles of ABA (abscisic acid), gene expression, and protein synthesis in facilitating acclimation to freezing temperatures.
Membrane Properties Change in Response to Chilling Injury
- Chilling injury in plants leads to a range of adverse effects on leaves, including the inhibition of photosynthesis, slower translocation of carbohydrates, reduced respiration rates, impaired protein synthesis, and increased degradation of existing proteins. These responses seem to share a common underlying mechanism, which involves the loss of membrane functionality during exposure to chilling temperatures.
- For example, when leaves from chilling-sensitive plants like Passiflora maliformis (conch apple) are placed in water at 0°C, solutes leak from the leaves. In contrast, chilling-resistant plants like Passiflora caerulea (passionflower) do not exhibit such solute leakage, indicating damage to the plasma membrane and possibly the tonoplast in chilling-sensitive plants. This damage, in turn, affects the functionality of chloroplast and mitochondrial membranes, leading to the inhibition of photosynthesis and respiration.

- The vulnerability of membranes to chilling is attributed to their composition. Plant membranes consist of a lipid bilayer interspersed with proteins and sterols. The physical properties of these lipids significantly influence the activities of integral membrane proteins involved in ion transport, solute transport, energy transduction, and enzyme-dependent metabolism.
- Chilling-sensitive plants tend to have a higher proportion of saturated fatty acids in their membrane lipids, causing their membranes to solidify into a semi-crystalline state at temperatures above 0°C. In contrast, membranes containing unsaturated fatty acids remain more fluid at lower temperatures. This increased fluidity allows for the normal functioning of membrane proteins. During acclimation to cooler temperatures, chilling-resistant plants increase the activity of desaturase enzymes and raise the proportion of unsaturated lipids in their membranes. This modification enables their membranes to maintain fluidity at lower temperatures, offering protection against chilling-induced damage.
- Experiments with mutant and transgenic plants have further highlighted the importance of membrane lipids in cold tolerance. For instance, when Arabidopsis plants were genetically modified to increase the proportion of high-melting-point (saturated) membrane lipids, they became more sensitive to chilling. Similarly, Arabidopsis mutants with elevated levels of saturated fatty acids, particularly 16:0, exhibited gradual inhibition of photosynthesis and growth when exposed to chilling temperatures, eventually leading to chloroplast destruction. These mutants performed as well as wild-type plants under nonchilling conditions.
Ice Crystal Formation and Protoplast Dehydration Kill Cells
- Tolerance to freezing temperatures varies across different plant tissues. Certain tissues like seeds, partially dehydrated tissues, and fungal spores can withstand extremely low temperatures, close to absolute zero (-273°C), without harm. This suggests that very low temperatures themselves are not inherently damaging.
- Fully hydrated vegetative cells can also survive freezing if they are rapidly frozen to prevent the formation of large, slow-growing ice crystals that could damage subcellular structures. The formation of small ice crystals during rapid freezing is not destructive. Similarly, quick thawing of frozen tissues is necessary to prevent small ice crystals from growing into damaging crystals or to avoid the loss of water vapor through sublimation, both of which occur at intermediate temperatures (-100 to -10°C).
- In natural conditions, the cooling of intact multicellular plant organs is typically not rapid enough to restrict ice formation within fully hydrated cells to small, harmless ice crystals. Ice usually forms first in the intercellular spaces and xylem vessels, where it can propagate rapidly. This ice formation is not lethal to hardy plants, and the tissue can recover when warmed. However, prolonged exposure to freezing temperatures can lead to the growth of extracellular ice crystals, which draws liquid water from the protoplast, causing excessive dehydration.
- During rapid freezing, the protoplast, including the vacuole, can supercool, meaning its cellular water remains in a liquid state even at temperatures well below its expected freezing point. Ice nucleation, the process by which ice crystals begin to form from hundreds of water molecules, depends on surface properties. Some large polysaccharides and proteins, known as ice nucleators, facilitate this process. In bacterial ice nucleation proteins, water molecules align along repeated amino acid domains within the protein. Within plant cells, ice crystals typically initiate from endogenous ice nucleators and can grow relatively large, leading to extensive cell damage and usually cell death.
Limitation of Ice Formation Contributes to Freezing Tolerance
- Certain specialized plant proteins, known as antifreeze proteins, play a role in limiting the growth of ice crystals during freezing by a mechanism that doesn't rely on the colligative effect (lowering of the freezing point due to solutes). These antifreeze proteins are induced by cold temperatures and function by binding to the surfaces of ice crystals, thereby preventing or slowing down their further growth.
- In rye leaves, antifreeze proteins are found in the epidermal cells and cells surrounding the intercellular spaces, where they can inhibit the growth of extracellular ice.
- Plants and animals may employ similar mechanisms to mitigate ice crystal growth. For instance, Arabidopsis possesses a cold-inducible gene that shares DNA homology with the gene responsible for encoding antifreeze proteins in certain fish species like winter flounder.
- Additionally, sugars and some cold-induced proteins are thought to have cryoprotective effects, stabilizing proteins and membranes during dehydration caused by low temperatures. In winter wheat, higher sucrose concentrations correlate with increased freezing tolerance. While sucrose is a prominent soluble sugar associated with freezing tolerance, other species may employ substances like raffinose, fructans, sorbitol, or mannitol for the same purpose.
- During the cold acclimation process of winter cereals, soluble sugars accumulate in the cell walls, potentially helping restrict ice growth. Cryoprotective glycoproteins, such as those isolated from cold-acclimated cabbage leaves, have demonstrated the ability to protect thylakoids (membranous structures involved in photosynthesis) from freezing-induced damage during in vitro experiments.
Some Woody Plants Can Acclimate to Very Low Temperatures
- Certain woody plants can develop a high degree of resistance to extremely low temperatures when they enter a dormant state. This resistance is influenced by both prior exposure to cold conditions and genetic factors that determine the extent of tolerance to low temperatures. For instance, native species of Prunus, which include cherry and plum trees, originating from cooler climates in North America tend to exhibit greater cold hardiness following acclimation compared to those from milder regions. Laboratory tests have highlighted distinct genetic differences among these species, with those from northern geographic distributions displaying a greater ability to prevent the formation of ice within their cells.
- In natural settings, woody plants undergo two distinct stages of acclimation to cold conditions, as described by Weiser (1970):
- First Stage: This initial stage of hardening occurs during early autumn when plants are exposed to short days and nonfreezing chilling temperatures, causing growth to cease. It's likely that a diffusible factor, potentially abscisic acid (ABA), is responsible for promoting this acclimation. During this period, these woody species also withdraw water from their xylem vessels to prevent stem splitting caused by water expansion during subsequent freezing. Although cells in this stage can survive temperatures well below freezing (0°C), they aren't completely hardened.
- Second Stage: The second stage involves direct exposure to freezing temperatures as the primary stimulus. In contrast to the first stage, there's no known translocatable factor that can induce hardening through exposure to freezing. When fully hardened during this stage, the cells can endure extremely low temperatures ranging from -50°C to -100°C.
Resistance to Freezing Temperatures Involves Supercooling and Slow Dehydration
- In various hardwood species found in southeastern Canada, the eastern United States, and mountainous regions like the Rocky Mountains of Colorado, adaptation to freezing conditions involves a unique phenomenon known as deep supercooling. This process enables these species to suppress the formation of ice crystals at temperatures significantly lower than the typical freezing point. Such deep supercooling is observed in trees like oak, elm, maple, beech, ash, walnut, hickory, rose, rhododendron, apple, pear, peach, and plum, as well as species such as Engelmann spruce and subalpine fir in the Rockies.
- The ability to resist freezing is most potent during the winter and diminishes as spring growth resumes. For instance, subalpine fir, which can withstand temperatures below -35°C in May, loses this resistance in June and can be damaged at -10°C.
- Deep supercooling has limits, with cells able to supercool only to approximately -40°C, beyond which ice formation becomes spontaneous. This phenomenon helps determine the minimum temperatures at which species that undergo deep supercooling can survive and contributes to the location of timberlines in mountain ranges, which often correspond to the -40°C isotherm.
- During deep supercooling, the cell protoplast prevents ice nucleation, and the cell wall acts as a barrier against ice growth from intercellular spaces into the wall and the loss of liquid water from the protoplast to extracellular ice.
- Several flower and vegetative buds, particularly in fruit trees like grape, blueberry, peach, azalea, apple, and pear, employ deep supercooling as a survival strategy over winter. However, the decline in freezing tolerance as buds transition to the spring can have economic consequences, as ice crystals forming in the bud scales during this period lead to water loss and ultimately dehydration of the floral apex, causing damage.
- In contrast, some woody species that endure extremely low temperatures, such as those in northern Canada, Alaska, northern Europe, and Asia, do not supercool but resist dehydration during extracellular ice formation. This resistance hinges on their capacity to accommodate growing ice crystals in extracellular spaces while simultaneously withstanding gradual protoplast dehydration.
- Interestingly, the ability to limit ice crystal formation to extracellular spaces, along with gradual protoplast dehydration, contributes to these species' resistance to water deficit during the growing season as well. For instance, species like willow, white birch, quaking aspen, pin cherry, chokecherry, and lodgepole pine can tolerate extremely low temperatures by restricting ice crystal formation to extracellular spaces. However, it's important to note that the acquisition of this resistance relies on slow cooling and the gradual formation of extracellular ice, and sudden exposure to very cold temperatures before full acclimation can lead to intracellular freezing and cell death.
Some Bacteria That Live on Leaf Surfaces Increase Frost Damage
- When leaves are exposed to temperatures ranging from -3°C to -5°C, the formation of ice crystals on their surfaces (known as frost) can be accelerated by certain bacteria that naturally inhabit the leaf surface. Examples of such bacteria include Pseudomonas syringae and Erwinia herbicola, which act as ice nucleators. When leaves are artificially inoculated with cultures of these bacteria, the leaves of frost-sensitive plant species freeze at higher temperatures compared to leaves that are free of these bacteria. This initiation of surface ice then rapidly spreads to the intercellular spaces within the leaf, ultimately resulting in cellular dehydration.
- To address this issue, researchers have developed genetically modified bacterial strains that lack the ice-nucleating characteristics. These modified strains have been used in commercial applications, particularly in foliar sprays for valuable frost-sensitive crops like strawberries. By introducing these modified strains, they compete with native bacterial strains on the leaf surfaces, effectively reducing the number of potential ice nucleation points and helping to protect the crops from frost damage.
ABA and Protein Synthesis Are Involved in Acclimation to Freezing
- Tolerance to freezing temperatures is influenced by the exposure to cold or abscisic acid (ABA) treatment in alfalfa seedlings. When subjected to cold (4°C) or treated with ABA without cold exposure, these seedlings exhibit changes in the pattern of newly synthesized proteins, as observed through two-dimensional gel analysis. Some protein changes are specific to each treatment (cold or ABA), but certain proteins induced by cold seem to overlap with those induced by ABA or mild water deficit stress.
- Protein synthesis is a crucial factor in developing freezing tolerance, and the process involves the accumulation of distinct proteins due to changes in gene expression. Some of these proteins share similarities with the RAB/LEA/DHN protein family, which are known to accumulate in response to various stresses like osmotic stress. The precise functions of these proteins are still under investigation.
- ABA appears to play a role in inducing freezing tolerance. Cold-tolerant species like winter wheat, rye, spinach, and Arabidopsis thaliana display increased freezing tolerance when subjected to water shortages, even at non-acclimating temperatures. This heightened freezing tolerance can also be achieved through mild water deficit or low temperatures, both of which elevate endogenous ABA levels in leaves.
- Plants can develop freezing tolerance at non-acclimating temperatures when treated with exogenous ABA. Many genes or proteins expressed at low temperatures or under water deficit conditions can also be induced by ABA at non-acclimating temperatures. This supports the idea that ABA is involved in freezing tolerance.
- However, not all genes induced by low temperatures are ABA-dependent, and it's not entirely clear if ABA-induced gene expression is essential for full freezing tolerance development. Some experiments with rye crowns showed that while ABA-treated crowns improved freezing tolerance, exposure to low temperatures had a more significant impact.
- In general, several days of exposure to cool temperatures are required for the full induction of freezing resistance, but this tolerance is rapidly lost upon rewarming. Therefore, plants that are not adequately acclimated can become vulnerable to freezing when temperatures drop suddenly.
- This phenomenon explains why plants in regions with highly variable winter temperatures, such as the southern United States, can be susceptible to extreme temperature fluctuations during the winter months, where temperatures can shift from mild to freezing within hours.
Numerous Genes Are Induced during Cold Acclimation
- During cold acclimation, numerous genes are induced, and specific proteins are synthesized in response to the cold stress. While there are some commonalities in gene expression and protein synthesis between heat and cold stress, there are also distinct differences.
- One significant difference is that during cold stress, the synthesis of "housekeeping" proteins, which are normally produced in the absence of stress, is not substantially reduced. In contrast, during heat stress, the synthesis of these housekeeping proteins is essentially shut down.
- However, both cold and heat stress result in the up-regulation of several heat shock proteins, which function as molecular chaperones. This suggests that protein destabilization occurs during both heat and cold stress, and mechanisms for stabilizing protein structure are crucial for survival in both situations.
- Another class of proteins induced by cold stress is antifreeze proteins. These proteins were initially discovered in fish living under polar ice caps and have the unique ability to inhibit ice crystal growth in a noncolligative manner, preventing freeze damage at intermediate freezing temperatures. Antifreeze proteins are sometimes referred to as thermal hysteresis proteins (THPs) because they promote the transition from liquid to solid at lower temperatures than the reverse transition from solid to liquid. Cold-acclimated winter-hardy monocots, such as some plants, produce various types of antifreeze proteins. Interestingly, these proteins are related to pathogenesis-related (PR) proteins, which protect plants against pathogens. In monocots, these proteins have a dual role, serving as antifreeze proteins and defending against pathogens, potentially safeguarding plant cells against both cold stress and pathogen attacks.
- Additionally, during cold stress, a group of proteins associated with osmotic stress is up-regulated. This group includes proteins involved in osmolyte synthesis, membrane stabilization, and LEA (late embryogenesis abundant) proteins. The formation of extracellular ice crystals during freezing stress generates significant osmotic stresses inside plant cells, making it essential to have mechanisms in place to cope with both freezing and osmotic stress.
A Transcription Factor Regulates Cold-Induced Gene Expression
- Cold stress leads to the up-regulation of over 100 genes, and many of these genes are likely associated with cold tolerance. The transcriptional activators responsible for regulating the expression of these cold stress-induced genes are known as C-repeat binding factors (CBF1, CBF2, and CBF3, also referred to as DREB1b, DREB1c, and DREB1a, respectively). These CBF/DREB1-type transcription factors play a crucial role in coordinating the transcriptional response to cold and osmotic stress.
- CBF/DREB1 transcription factors bind to CRT/DRE elements (C-repeat/dehydration-responsive, ABA-independent sequence elements) found in the promoter regions of target genes. These elements were discussed earlier in the chapter. CBF/DREB1 factors are involved in the transcriptional regulation of many genes that respond to cold and osmotic stress. Importantly, all of these genes contain CRT/DRE elements in their promoters.
- CBF1/DREB1b is unique in that it is specifically induced by cold stress and not by osmotic or salinity stress. In contrast, there are DREB2-type factors that are induced by osmotic and salinity stress but not by cold.
- The expression of CBF1/DREB1b is controlled by a separate transcription factor known as ICE (inducer of CBF expression). ICE transcription factors are not induced by cold itself, and it is believed that ICE or an associated protein is posttranscriptionally activated, allowing the activation of CBF1/DREB1b. However, the specific signaling pathways responsible for cold perception, calcium signaling, and ICE activation are still under investigation.
- Transgenic plants that constitutively express CBF1 produce more transcripts of cold-up-regulated genes, even in the absence of cold stress. This suggests that numerous proteins involved in cold acclimation are being produced in these CBF1 transgenic plants without the need for cold induction. Furthermore, these CBF1 transgenic plants exhibit increased cold tolerance compared to control plants, indicating the significant role of CBF1 in enhancing cold tolerance.
Salinity Stress
- In their natural habitats, terrestrial higher plants often encounter high salt concentrations in areas near the seashore, estuaries where freshwater mixes with seawater, and regions with salt seepage from geological marine deposits. These conditions can render the land unsuitable for agriculture. However, a more significant agricultural problem is the accumulation of salts from irrigation water.
- The process of evaporation and transpiration removes pure water as vapor from the soil, leading to the concentration of solutes in the soil. When irrigation water contains a high concentration of solutes and there is no effective drainage system to flush out accumulated salts, salt levels in the soil can quickly rise to levels that are harmful to salt-sensitive plant species. Approximately one-third of the irrigated land worldwide is affected by soil salinity issues.
- In the following section, we will explore how plant function is impacted by water and soil salinity, as well as the mechanisms that plants use to cope with and avoid salinity stress.

Salt Accumulation in Soils Impairs Plant Function and Soil Structure
- When discussing the impact of salts in the soil, we differentiate between high concentrations of Na+ ions, referred to as sodicity, and high concentrations of total salts, which we refer to as salinity. These two concepts are often related, but in some regions, other ions such as Ca2+, Mg2+, SO2 4–, in addition to NaCl (common table salt), can significantly contribute to salinity. High Na+ concentration in sodic soil not only directly harms plants but also degrades soil structure, reducing porosity and water permeability. Some sodic clay soils, like caliche, are so dense and impermeable that they may require dynamite for excavation.
- In the field, the salinity of soil water or irrigation water is typically measured in terms of its electrical conductivity or osmotic potential. Pure water is a poor conductor of electric current, and the conductivity of a water sample is a result of the ions dissolved in it. The higher the salt concentration in water, the greater its electrical conductivity and the lower its osmotic potential (higher osmotic pressure).
- In semiarid and arid regions, the quality of irrigation water is often poor. For example, the salt content of the Colorado River's headwaters in the United States is only 50 mg L–1, but as the river flows about 2000 km downstream to southern California, the salt content increases to about 900 mg L–1, which can hinder the growth of salt-sensitive crops like maize. Some wells used for irrigation in Texas may contain as much as 2000 to 3000 mg of salt per liter of water. If 1 meter of irrigation water is applied annually from such wells, it would add 20 to 30 tons of salts per hectare (8–12 tons per acre) to the soil. These salt levels are detrimental to most crops, except those highly resistant to salinity.
Salinity Depresses Growth and Photosynthesis in Sensitive Species
- Plants can be categorized into two main groups based on how they respond to high salt concentrations. Halophytes are plants naturally adapted to saline soils, and they complete their life cycles in such environments. Glycophytes, on the other hand, are plants that cannot withstand high salt levels to the same extent as halophytes. Typically, glycophytes start displaying signs of inhibited growth, leaf discoloration, and reduced dry weight when salt concentrations exceed a certain threshold.
- Within cultivated crops, certain plants like maize, onion, citrus, pecan, lettuce, and beans are highly sensitive to salt stress. Cotton and barley exhibit moderate tolerance, while sugar beet and date palms are highly salt-tolerant. Remarkably, some species with high salt tolerance, such as Suaeda maritima (a salt marsh plant) and Atriplex nummularia (a saltbush), can even experience growth stimulation at chloride ion (Cl–) concentrations much higher than those lethal to sensitive species (as shown in Figure 25.14).
Salt Injury Involves Both Osmotic Effects and Specific Ion Effects
- Excessive salt levels in the soil create a low osmotic potential due to the presence of dissolved solutes. This low osmotic potential reduces the overall water potential in the soil, which in turn affects the water balance in plants. To maintain a favorable gradient of water potential between the soil and the leaves, plants must develop an even lower water potential in their leaves. This response to soil salinity is similar to how plants react to water deficits, as discussed earlier.
- One notable distinction between the effects of soil salinity and soil desiccation (water deficit) is the total amount of available water. In saline environments, there is often a large and essentially unlimited supply of water at a consistently low water potential, whereas water availability decreases progressively in desiccated soils.
- Most plants have the ability to adjust osmotically when growing in saline soils, which helps them prevent turgor loss (a condition that slows cell growth). However, these plants often continue to grow more slowly after osmotic adjustment, for reasons that are not yet fully understood.
- In addition to the osmotic effects, specific ion toxicity comes into play when harmful concentrations of certain ions, particularly Na+, Cl–, or SO4^2–, accumulate in plant cells. Under normal conditions, the cytosol of plant cells contains around 100 to 200 mM K+ and 1 to 10 mM Na+. This ionic balance is essential for the optimal function of many enzymes. When there is an abnormally high Na+/K+ ratio or elevated total salt concentrations, enzymes become inactivated, and protein synthesis is inhibited. High levels of Na+ can displace Ca2+ from the plasma membrane, altering its permeability and leading to the leakage of K+ from the cells. Chloroplasts are also affected by the accumulation of Na+ and Cl–, which inhibits photosynthesis, possibly by affecting carbon metabolism or photophosphorylation.
- It's important to note that enzymes extracted from salt-tolerant plants are just as sensitive to the presence of NaCl as enzymes from salt-sensitive glycophytes. Therefore, the resistance of halophytes (salt-tolerant plants) to salt injury is not due to having inherently salt-resistant metabolic processes. Instead, they employ other mechanisms to avoid salt damage, which will be discussed in the following section.

Plants Use Different Strategies to Avoid Salt Injury
- Plants employ various strategies to minimize salt injury, particularly in the shoot and actively expanding and photosynthesizing leaves. For salt-sensitive plants, their resistance to moderate soil salinity relies partly on the roots' ability to prevent harmful ions from reaching the shoots.
- The Casparian strip, discussed in Chapter 4, serves as a barrier to ion movement into the xylem. To bypass the Casparian strip, ions must transition from the apoplast to the symplastic pathway across cell membranes. This transition allows salt-resistant plants to partially exclude harmful ions.
- Sodium ions (Na+) enter roots passively due to electrochemical potential gradients, requiring energy for active extrusion back to the external solution. Chloride ions (Cl–) are excluded by negative electric potential across the cell membrane and the low permeability of root plasma membranes to this ion. Moreover, Na+ movement into leaves is further minimized by the absorption of Na+ from the transpiration stream (xylem sap) during its transport from roots to shoots and leaves.
- Certain salt-resistant plants, such as salt cedar (Tamarix sp.) and salt bush (Atriplex sp.), have salt glands on their leaf surfaces. These glands transport ions to their surfaces, where the salt crystallizes and is no longer harmful. Halophytes (salt-tolerant plants) generally have a greater capacity than glycophytes (salt-sensitive plants) for ion accumulation in shoot cells.
- Some plants, like mangroves, thrive in saline environments with ample water supplies. However, obtaining water in such environments requires osmotic adjustments to access water from the low-water-potential external environment. Plant cells can adjust their water potential (Yw) by lowering their solute potential (Ys).
- This reduction in Ys involves two processes:
- Accumulation of ions in the vacuole.
- Synthesis of compatible solutes in the cytosol. Compatible solutes include glycine betaine, proline, sorbitol, mannitol, pinitol, and sucrose. Different plant families tend to prefer specific compatible solutes. The synthesis of these organic solutes may divert a significant portion of carbon (about 10% of plant weight) for adjusting water potential. While this adjustment is generally well-tolerated in natural vegetation, it can reduce growth, biomass, and yield in agricultural crops.
- Many halophytes exhibit an optimal growth rate at moderate salinity levels, and this growth is associated with their ability to accumulate ions in the vacuole, contributing to cell osmotic potential without harming salt-sensitive enzymes. Salt-sensitive glycophytes can also undergo this process, although their adjustment may be slower.
- In addition to adjusting water potential, plants dealing with salinity stress undergo other acclimations related to osmotic stress, such as reducing leaf area or shedding leaves through abscission, just as they do during episodes of water deficit. Furthermore, changes in gene expression linked to osmotic stress are similarly associated with salinity stress. It's important to note that in salinity stress, plants must also manage the toxicity resulting from high ion concentrations.
Ion Exclusion Is Critical for Acclimation and Adaptation to Salinity Stress
- Utilizing ions to balance tissue water potential in a saline environment is more energy-efficient for plants compared to using carbohydrates or amino acids, which have a significantly higher energy cost. However, the presence of high ion concentrations can be toxic to many cytosolic enzymes. Therefore, plants must accumulate these ions in the vacuole to minimize toxic concentrations in the cytosol.
- Since sodium chloride (NaCl) is the most common salt encountered by plants during salinity stress, transport systems that facilitate the compartmentalization of sodium ions (Na+) into the vacuole are crucial. Both calcium ions (Ca2+) and potassium ions (K+) influence intracellular Na+ concentrations.
- Under high concentrations of Na+, the uptake of K+ through a high-affinity K+–Na+ transporter known as HKT1 is inhibited, causing the transporter to function as an Na+ uptake system instead. Calcium, on the other hand, enhances the selectivity of K+ over Na+ (K+/Na+ selectivity), thereby increasing salt tolerance. This selectivity is critical for maintaining proper ion balance and preventing sodium toxicity in plant cells.
Sodium Is Transported across the Plasma Membrane and the Tonoplast
- The transport of sodium ions (Na+) across the plasma membrane and the tonoplast (vacuolar membrane) is facilitated by proton pumps (H+-ATPases) that create electrochemical potential gradients for hydrogen ions (H+) across these membranes. These proton pumps play a crucial role in providing the energy required for secondary ion transport in response to salinity stress.
- Plasma Membrane: An ATPase primarily generates the large ∆pH (difference in pH) and membrane potential gradient across the plasma membrane. This ATPase creates an electrochemical potential gradient, which serves as the driving force for the secondary transport of ions. The activity of these H+ pumps increases under salinity stress conditions. ATPases are regulated by gene expression, and some of this upregulation may be attributed to induced gene expression in response to salt stress.
- Tonoplast (Vacuolar Membrane): A vacuolar H+-ATPase generates a ∆pH and membrane potential across the tonoplast. This tonoplast ATPase is responsible for creating an electrochemical potential gradient across the vacuolar membrane.
- Energy-dependent transport (efflux) of Na+ from the cytosol of plant cells across the plasma membrane is mediated by the product of the SOS1 (Salt Overly Sensitive 1) gene. SOS1 functions as a Na+-H+ antiporter, transporting Na+ ions out of the cell in exchange for H+ ions. This process is critical for maintaining ion homeostasis under salinity stress conditions. The activity of SOS1 is regulated by the gene products of SOS2 and SOS3, with SOS2 acting as a serine/threonine kinase and SOS3 serving as a calcium-regulated protein phosphatase. Calcium signaling plays a role in activating SOS2 through SOS3.
- The compartmentation of Na+ ions into vacuoles is facilitated, in part, by a family of Na+-H+ antiporters, such as AtNHX1 in Arabidopsis. These antiporters are responsible for transporting Na+ ions into the vacuole, sequestering them away from the cytosol, and contributing to salt tolerance in plants. Transgenic plants overexpressing genes like AtNHX1 have been found to exhibit enhanced salt tolerance.
- These molecular insights into ion transport and compartmentation mechanisms have been uncovered through the study of transgenic plants, gene sequencing, and protein characterization, contributing to our understanding of how plants cope with salinity stress.


Oxygen Deficiency
- Roots typically receive sufficient oxygen (O2) for aerobic respiration from the air-filled spaces in the soil. Well-drained and structured soils allow the gaseous O2 to diffuse deep into the soil, maintaining O2 concentrations similar to those in the surrounding air. However, problems can arise when soil becomes waterlogged or flooded due to poor drainage or excessive precipitation or irrigation. In such cases, water fills the soil pores, hindering the diffusion of gaseous O2. The slow diffusion of dissolved oxygen in stagnant water means that only the top few centimeters of soil near the surface remain oxygenated.
- In cold weather when plants are dormant, oxygen depletion in the soil is gradual and less harmful. However, at higher temperatures (above 20°C), the consumption of oxygen by plant roots, soil organisms, and microorganisms can deplete the oxygen from the soil water within as little as 24 hours.
- Plants that are sensitive to flooding can be severely damaged within 24 hours of oxygen deprivation. Many plant species experience reduced growth and survival under such conditions, leading to significant reductions in crop yields. For instance, garden peas are highly sensitive to flooding, and their yields can be cut in half after just 24 hours of oxygen deprivation.
- Other plants, especially those not adapted to continuous wet conditions and many crop plants, are affected by flooding to a lesser extent and are considered flooding-tolerant. These flooding-tolerant plants can endure a temporary lack of oxygen but cannot survive for extended periods of more than a few days without oxygen.
- In contrast, specialized vegetation found in wetlands like marshes and swamps, as well as crops like rice, have evolved to thrive in oxygen-deprived root environments. These wetland plants can resist anoxia and can grow and survive for extended periods, even months, with their roots in oxygen-deprived conditions. Most of these plants possess specific adaptations that allow them to access oxygen from nearby oxygenated environments. While all plants need oxygen during periods of rapid metabolic activity, they can be categorized based on their ability to withstand anoxic conditions in their root environments without significant damage.
- In the subsequent sections, we will explore the damage inflicted by anaerobic (oxygen-deprived) conditions on both roots and shoots, the strategies employed by wetland vegetation to cope with low oxygen levels, and the various adaptations to anoxic stress that differentiate between flooding-tolerant and flooding-susceptible plant species.
Anaerobic Microorganisms Are Active in Water-Saturated Soils
- In situations where soil is completely devoid of molecular oxygen (O2), the activity of anaerobic microorganisms plays a crucial role in shaping the conditions for plant life and growth. These anaerobic microorganisms, known as anaerobes, obtain their energy through processes like the reduction of nitrate (NO3-) to nitrite (NO2-) or to nitrous oxide (N2O), ultimately leading to the formation of molecular nitrogen (N2). These gases, particularly N2O and N2, are released into the atmosphere in a process called denitrification.
- As conditions in the soil become increasingly reducing (lacking in oxygen), anaerobic microorganisms may also engage in the reduction of ferric iron (Fe3+) to ferrous iron (Fe2+). This process can lead to the accumulation of toxic levels of soluble Fe2+ in the soil, especially when the soil remains anaerobic for extended periods.
- Additionally, certain anaerobic microorganisms have the capacity to reduce sulfate ions (SO42-) to hydrogen sulfide (H2S), which is a respiratory poison. When anaerobes have an ample supply of organic substrates, they release metabolic byproducts such as acetic acid and butyric acid into the soil water. These acids, along with reduced sulfur compounds, are responsible for the unpleasant odor often associated with waterlogged soil. It's important to note that these substances, when produced in high concentrations by microorganisms under anaerobic conditions, can be toxic to plants.
Roots Are Damaged in Anoxic Environments
- Roots are particularly vulnerable to damage in anoxic environments, where oxygen levels are severely limited. Even before oxygen is completely depleted from the root environment, root respiration rate and metabolism are affected. The critical oxygen pressure (COP) is a key parameter, representing the oxygen pressure at which the respiration rate of roots is first slowed due to oxygen deficiency.
- For example, in maize root tips growing in well-stirred nutrient solutions at 25°C, the COP is approximately 0.20 atmosphere (20 kPa or 20% oxygen by volume). At this oxygen partial pressure, the rate of oxygen diffusion from the surrounding solution into the root tissue barely matches the rate of oxygen utilization. It's worth noting that root tips are metabolically highly active, with respiration rates and ATP turnover comparable to those of mammalian tissues.
- In contrast, in older sections of the root where cells are mature, fully vacuolated, and have lower respiration rates, the COP is typically in the range of 0.1 to 0.05 atmosphere. When oxygen concentrations drop below the COP, the center of the root can become either anoxic (completely devoid of oxygen) or hypoxic (partially deficient in oxygen).
- The COP can vary based on factors such as temperature, the size of the organ, and how densely packed the cells are. Larger, bulkier organs like fruits can remain aerobic because of their intercellular spaces, which allow for efficient gaseous diffusion. In contrast, single cells can sustain their functions with even lower oxygen partial pressures, sometimes as low as 0.01 atmosphere (1% oxygen in the gaseous phase), thanks to the short distances required for oxygen diffusion to reach the mitochondria.
- In the absence of sufficient oxygen for aerobic respiration, roots resort to fermentation, particularly lactate fermentation. However, lactate fermentation is a less efficient process, yielding only a fraction of the ATP produced in aerobic respiration. This oxygen deficiency-induced shift in metabolism ultimately results in a lack of ATP to drive vital metabolic processes.
- In cases of extreme oxygen deficiency, intracellular pH levels can become disrupted. For instance, protons may leak from the vacuole into the cytoplasm, leading to cytosolic acidosis. This shift in pH is associated with cell death. The disruption of metabolism in the cytoplasm due to cytosolic acidosis is a primary factor distinguishing plants that are sensitive to flooding from those that are tolerant of it. Flooding-tolerant species are better equipped to manage and limit the effects of cytosolic acidosis.

Damaged O2-Deficient Roots Injure Shoots
- Oxygen-deficient roots, whether anoxic or hypoxic, lack the necessary energy to support essential physiological processes that the shoots rely on. Experimental studies have demonstrated that when the roots of plants like wheat or barley are unable to absorb nutrient ions and transport them to the xylem (and subsequently to the shoots), it results in a rapid shortage of ions within developing and expanding tissues. This ion deficiency can lead to premature senescence of older leaves because essential elements such as nitrogen (N), phosphorus (P), and potassium (K) are reallocated to younger leaves. Additionally, the reduced permeability of oxygen-deficient roots to water can cause a temporary decrease in leaf water potential and wilting, although this decrease is reversible if stomata close, preventing further water loss through transpiration.
- Hypoxia (low oxygen levels) in roots can also accelerate the production of the precursor of the plant hormone ethylene, known as ACC (1-aminocyclopropane-1-carboxylic acid). In plants like tomato, ACC is transported through the xylem sap to the shoot, where it is converted to ethylene by ACC oxidase in the presence of oxygen. Ethylene-responsive cells on the upper (adaxial) surfaces of leaf petioles in tomato and sunflower then expand more rapidly when ethylene concentrations are high. This rapid expansion results in epinasty, where the leaves appear to droop or bend downward. Unlike wilting, epinasty does not involve a loss of turgor pressure.
- In certain plant species such as peas and tomatoes, flooding can induce stomatal closure even when there are no detectable changes in leaf water potential. The oxygen deficiency in the roots, similar to water deficit or high salt concentrations, can stimulate the production of abscisic acid (ABA) and its movement to the leaves. However, under these conditions, stomatal closure is primarily attributed to the additional production of ABA by the older, lower leaves that are wilting. These older leaves export their ABA to the younger, turgid leaves, leading to stomatal closure and a reduction in water loss through transpiration.
Submerged Organs Can Acquire O2 through Specialized Structures
- Wetland vegetation has evolved adaptations that enable it to thrive in water-saturated soil, even when portions of the plants are submerged. These adaptations ensure vigorous growth without signs of stress.
- Several mechanisms are employed by wetland plants to cope with submergence:
- Ethylene Response: Some wetland species, like the water lily (Nymphoides peltata), can trap endogenous ethylene, a plant hormone, which stimulates the rapid elongation of petioles. This elongation allows the leaves to reach the water's surface and access the air. Deep-water (floating) rice also responds to trapped ethylene by extending its leaves above the water's surface, despite increasing water depth. Pondweed (Potamogeton pectinatus) promotes stem elongation through water acidification caused by respiratory CO2 accumulation.
- Gas-Filled Channels: In many wetland plants, both stems and roots develop longitudinally interconnected gas-filled channels. These channels provide a low-resistance pathway for the movement of oxygen and other gases. Gases enter through stomata or lenticels on woody stems and roots and move via molecular diffusion or convection driven by small pressure gradients.
- Aerenchyma Formation: Wetland plants often develop a tissue called aerenchyma, characterized by prominent, gas-filled spaces between cells. Aerenchyma facilitates the movement of oxygen and gases within the plant. In some nonwetland plants, such as both monocots and dicots, aerenchyma formation is induced by oxygen deficiency in the stem base and developing roots.
- Ethylene-Mediated Cell Death: Under oxygen-deficient conditions, hypoxia can stimulate the production of ethylene in the root tip of certain plants like maize. Ethylene triggers cell death and disintegration in the root cortex. The spaces created by these disintegrating cells provide gas-filled voids that facilitate oxygen movement.
- Continuous Aerenchyma Formation: In plants like rice, aerenchyma continuously forms just behind the root tip. This ongoing formation ensures that oxygen is supplied to the apical zone as the root extends into oxygen-deficient soil. Structural barriers in the roots prevent the outward diffusion of oxygen, conserving it for the apical meristem and allowing roots to grow deep into anaerobic soil.
- These adaptations collectively enable wetland plants to withstand and thrive in waterlogged environments by ensuring an adequate supply of oxygen to vital plant tissues, even when submerged or growing in oxygen-deficient conditions.

Most Plant Tissues Cannot Tolerate Anaerobic Conditions
- Most tissues of higher plants are unable to survive prolonged anaerobic (oxygen-deficient) conditions. For instance, the root tips of maize can only remain viable for about 20 to 24 hours if they are suddenly deprived of oxygen. During anaerobic conditions, some ATP can be generated slowly through fermentation, but over time, the energy status of cells gradually declines due to cytosolic acidosis. The specific combination of biochemical characteristics that allows some cells to tolerate anoxia (lack of oxygen) for extended periods is not yet fully understood.
- In the case of root tips of maize and other cereals, they exhibit a limited degree of acclimation if they are first exposed to hypoxic conditions, which enables them to survive up to 4 days of anoxia. This acclimation is associated with the activation of genes responsible for anaerobic stress responses. After acclimation, these cells show an improved ability to carry out ethanolic fermentation under anoxia (producing ATP to sustain some metabolism) and transport lactate out of the cytosol to the external medium, thereby minimizing cytosolic acidosis.
- The ability of organs in wetland plants to tolerate chronic anoxia likely relies on similar strategies but is more effective. Critical factors include control of cytosolic pH, continued ATP generation through glycolysis and fermentation, and sufficient fuel storage for anaerobic respiration over extended periods. Some studies suggest that the synthesis of compounds like alanine, succinate, and γ-aminobutyric acid during anoxia helps consume protons and minimize cytosolic acidosis. This mechanism has been observed in anoxia-tolerant shoots of rice and rice grass but not in anoxia-sensitive shoots of wheat and barley.
- For plants that alternate between anaerobic and aerobic metabolism, dealing with the consequences of reoxygenation (entry of oxygen) after anoxia is crucial. Reactive oxygen species are generated during aerobic metabolism, and these are typically detoxified by cellular defense mechanisms involving enzymes like superoxide dismutase (SOD). SOD converts superoxide radicals into hydrogen peroxide, which is then further converted into water by peroxidase. In anoxia-tolerant rhizomes of Iris pseudacorus (yellow flag), SOD activity increases significantly during prolonged anoxia, which may help these plants cope with the influx of oxygen when they reemerge into the air from water or mud, thus assisting in resisting post-anoxic stress.
Acclimation to O2 Deficit Involves Synthesis of Anaerobic Stress Proteins
- When maize roots are exposed to anoxic (oxygen-deficient) conditions, most protein synthesis ceases, except for the continued production of about 20 specific polypeptides known as anaerobic stress proteins. These proteins have been identified as enzymes involved in the glycolytic and fermentation pathways, which are crucial for anaerobic metabolism.
- The exact mechanism by which plant cells sense reduced oxygen levels under hypoxic or anoxic conditions is not fully understood. However, one of the earliest events following a decrease in oxygen levels is an elevation of intracellular calcium ion (Ca2+) concentration. This rise in Ca2+ concentration is believed to be a signal involved in the response to anoxia. Within minutes of exposure to anoxia, this increase in cytosolic Ca2+ concentration serves as a signal that leads to an upregulation of mRNA levels for enzymes like alcohol dehydrogenase (ADH) and sucrose synthase in maize cells cultured under anoxic conditions.
- Chemicals that prevent the rise in intracellular Ca2+ concentration also block the induction of genes for ADH and sucrose synthase under anoxic conditions. Additionally, these chemicals make maize seedlings more sensitive to anoxia. However, the precise mechanisms of how intracellular Ca2+ concentration is sensed, how it leads to alterations in the transcription of specific genes, and how it controls both the early survival of cells under anoxia and the induction of processes like cell death and aerenchyma formation during prolonged hypoxia require further research.
- The accumulation of mRNAs for anaerobic stress genes is a result of changes in the transcription rate of these genes. Studies have identified common sequence elements in the promoters of anaerobic stress genes, such as ADH genes in maize and Arabidopsis, including an anaerobic stress element and a G-box element. These elements bind cis-acting transcription factors, leading to the transcriptional activation of these genes. However, the exact details of how oxygen deficiency is sensed, how the signal is transduced through increases in cytosolic Ca2+, and how this leads to changes in the transcription of specific genes remain to be fully elucidated.
- It's worth noting that there is also evidence suggesting that translational control of anaerobic stress genes may be occurring. The efficiency with which mRNAs for genes not regulated by anaerobic stress are translated following hypoxic stress is significantly lower compared to the translation of stress-regulated genes like ADH.
Summary
In summary, plants often face various forms of stress in their natural environments, and their ability to cope with these stresses is known as stress resistance. Key stress factors include:
- Water Deficit: Plants adapt to water deficit through mechanisms like leaf abscission, root extension into deeper soil, and stomatal closure. Genes involved in acclimation to water stress mediate these responses, with both ABA-dependent and ABA-independent pathways playing crucial roles.
- Heat Stress: High temperatures can lead to heat stress, damaging plant tissues and impairing photosynthesis. Plants use adaptations like leaf rolling, reduced leaf size, and the synthesis of heat shock proteins to resist heat stress.
- Chilling and Freezing Stress: Chilling injury occurs at temperatures above freezing but too low for normal growth. Freezing injury results from ice crystal formation within cells and organs. Plants resist freezing by limiting ice crystal growth in extracellular spaces, while chilling-resistant plants often have membrane lipids with more unsaturated fatty acids.
- Salinity Stress: Salinity stress arises from the accumulation of salts in the soil, which can depress plant growth and photosynthesis. Plants avoid salt injury by excluding excess ions from leaves or compartmentalizing ions in vacuoles. The SOS pathway regulates genes involved in ion homeostasis.
- Oxygen Deficiency: Oxygen deficiency in flooded or waterlogged soils can harm plant growth and survival. Some tissues, like the embryo and coleoptiles of rice, can survive under anoxic conditions. Plants have metabolic pathways to resist anoxic damage, involving the regulation of genes.
In each case, plants exhibit various adaptations and physiological responses to minimize the negative effects of stress. These adaptations are often genetically determined, but acclimation, which occurs when plants are exposed to stress beforehand, can also improve their resistance. Stress resistance mechanisms are crucial for plants to reach their genetic potential and thrive in challenging environments.
|
179 videos|140 docs
|

|
Explore Courses for UPSC exam
|

|


















