Structural Isomerism | Chemistry Class 11 - NEET PDF Download
| Table of contents |

|
| What is Isomerism? |

|
| What is Structural isomerism? |

|
| Chain Isomerism |

|
| Position Isomerism |

|
| Functional Group Isomerism |

|
| Metamerism |

|
| Tautomerism |

|
| Ring-chain Isomerism |

|
What is Isomerism?
Compounds having the same molecular formula but different properties are known as isomers and this phenomenon is known as isomerism.
The term "isomer" comes from the Greek words "isos" and "meros," which together mean "equal parts." A Swedish chemist named Jacob Berzelius created this term in 1830.
Classification of Isomerism
Two common types of isomerism are structural isomerism and stereoisomerism.
- Structural Isomerism: In structural isomerism, molecules with identical molecular formulas have different arrangements of atoms. This can manifest as variations in the branching of carbon chains, positions of functional groups, or the presence of different functional groups.
- Stereoisomerism: Stereoisomerism, on the other hand, involves molecules with the same connectivity of atoms but differ in their spatial arrangement. This can occur as geometric isomerism, where groups around a double bond have different spatial orientations, or optical isomerism, where molecules are mirror images of each other but cannot be superimposed. These isomers can exhibit unique properties due to their distinct spatial structures.
 Classification of Isomerism
Classification of Isomerism
What is Structural isomerism?
- Compounds having the same molecular formula and different connectivity of atoms (Structure is different) are called Structural Isomers.
- A straightforward example is the alkane with a molecular formula of C4H10, showcasing structural isomers with varied arrangements. As the number of carbon atoms in the alkane molecule increases, the number of structural isomers also increases.
 Structural Isomers of Butane
Structural Isomers of Butane
Classification of Structural Isomers
There are six types of Structural Isomers:
- Chain Isomers: These isomers have the same molecular formula but differ in the arrangement of the carbon skeleton, creating distinct chains or branches.
- Position Isomers: In this type, atoms are attached at different positions on the carbon chain, leading to unique compounds with distinct physical and chemical properties.
- Functional Group Isomers: Molecules with the same molecular formula but different functional groups fall under this category. For example, an alcohol and an ether can be functional group isomers.
- Tautomers: Tautomeric isomers exist in equilibrium with each other due to the migration of a hydrogen atom or a proton. They rapidly interconvert, creating dynamic structures.
- Metamers: Metamers have the same alkyl groups on either side of a functional group, such as an ether or a secondary amine, but with a different arrangement of alkyl groups.
- Ring Isomers: These isomers involve variations in the arrangement of atoms to form different cyclic structures while maintaining the same molecular formula. The shift from one ring structure to another characterizes this type of isomerism.
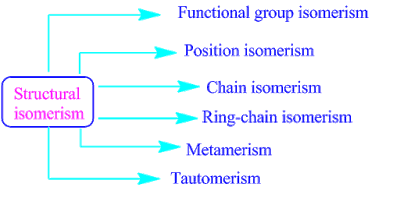 Classification of Structural Isomers
Classification of Structural Isomers
Chain Isomerism
Compounds having the same molecular formula but differ in the length of the principal chain.

How to Form Chain Isomers?
CnH2n+2 CnH2n+1
CnH2n+1
Alkane Alkyl group


dark line (-) represents vacant valency where any group can be attached.
- Iso-Group
e.g.Isoheptane

e.g. Isooctane (exception for the Iso group)

- Neo Group

To prepare the neo compound firstly the above group is written. After that required no. of carbon is added in the straight chain.
is written. After that required no. of carbon is added in the straight chain.
e.g. neopentane

e.g. neo heptane

Examples of Chain Isomerism:
1. C4H10 has 4 forms


2. C5H12 has it's three forms:
C - C - C - C - C
(n-pentane)

Consider n-pentane:

= 3 form
Consider Isopentane,

Consider neopentane,

= only one form.
Total forms of C5H11 - = 8
3. CH3 - CH2 - CH2 - CH3 - butane (n-butane)
and
 - 2-methylpropane (Isobutane)
- 2-methylpropane (Isobutane)
4.  - butanoic acid and
- butanoic acid and
 - 2-methyl propanoic acid
- 2-methyl propanoic acid
Ex.1 Find all the structural isomers of C6H14
Sol.
 Structural Isomers of C6H14
Structural Isomers of C6H14
Position Isomerism
Compounds having the same molecular formula and same principal chain but differ in the position of functional group, multiple bonds, and substitution group are known as position isomers.
 Position Isomerism
Position Isomerism
e.g. C - C - C = C (1-butene) and C - C = C - C ( 2-butene)
e.g.  (1-chloropropane) and
(1-chloropropane) and  (2-chloropropane) are position isomers.
(2-chloropropane) are position isomers.
e.g.  (1-propanol) and
(1-propanol) and  (2-propanol)
(2-propanol)
Note:
and
are chain isomers, not position isomers.
The above example can be best understood taking the following example
C - C - C - C and
In this, the last carbon has been placed to IInd position to form chain isomer the same has happened with above example and hence they are chain isomer to each other.
Ex.2 Find the relation between the given compounds:
(A) C - C - C - C - C - C
(B)
(C) 
(D) 
(E) 
Sol. a, b →chain isomers. b, c →position isomers.
c, d →chain isomers. d, e →position isomers.
Ex.3 How many monochloro derivatives will be of C4H10 (Only structural)
Sol.
 Monochloro Derivative of C4H10
Monochloro Derivative of C4H10
Important: Monochlorination →Replace one H by Cl
Ex.4 An alkane having molecular formula C5H12 can give only one product on monochlorination. Find the IUPAC name of the alkane.
Sol.
(2, 2-dimethyl pentane)
Ex.5
 Mono chlorinated products (Excluding Stereoisomers)?
Mono chlorinated products (Excluding Stereoisomers)?
Sol.



Ex.6 Find the total structural isomer of C5H10.
Sol. To solve these kinds of problems we should at once draw all possible structures of corresponding alkanes and then we should check how many possibilities are there to put a double bond.
 = 2
= 2
 = 3
= 3
 no double bond can be placed in this compound as the valency of C will exceed from 4
no double bond can be placed in this compound as the valency of C will exceed from 4
Total open chain structural isomers = 5
* To form a cyclic structure we should always start with 3 carbon ring.





total structural isomers = 5 + 5 = 10
Ex.7 Find the total structural isomers of C4H6.
Sol. Total unsaturation of C4H6 = 2
i.e. possibility = one triple bond, or 2 double bonds, or (one ring + one double bond)
 ( indicates the possible position of triple bond)
( indicates the possible position of triple bond)
 → (No triple bond)
→ (No triple bond)
for alkene,

total open chain = 2 + 2 = 4
for cyclic 




total structural isomers (cyclic, acyclic) = 9
Ex.9 Find all the structural dichloro derivatives of cyclopentane.
Sol.



Total structural isomer = 3
Functional Group Isomerism
Compounds having same molecular formula but different in functional group are known as functional isomers.

Important:
- Aldehyde, Ketone, cyclic ethers, cyclic alcohol, unsaturated alcohol etc. are functional isomers to each other.
- Alcohol and ether are functional isomers to each other. e.g. CH3CH2OH, CH3OCH3
- Acids and esters are functional isomers to each other. eg.
- Cyanide and Isocyanide are functional isomers to each other but HCN and HNC are tautomers to each other.
- * 1°, 2° and 3° amine are functional isomers to each other.
e.g. C3H6O CH3 - CH2 CHO  CH2 = CH - CH2OH
CH2 = CH - CH2OH

Ex.10 How many primary, secondary, and tertiary alcohols are possible for C5H13O?(Only structural)
Sol. For 1° alcohol (-CH2OH)
C5H12OH ⇒ C4H9 - CH2 - OH (4 form)
for 2° alcohol  →
→ 
Replace one C (1 form)
total = 3
for 3° alcohol 
total = 4 + 3 + 1 = 8
or C5H13O ⇒ C5H12 - OH (8 form)
Ex.11 How many ethers are possible in C5H12O?(Only structural).
Sol. C4H9OCH3 ⇒ C3H7OC2H5
(4 form) (2 form)
total = 6
Ex.12 How many 1º, 2º, and 3º amine are possible for C5H13N(Only structural).
Sol. For 1º amine (- NH2)
C5H11 NH2 ⇒ (8 form) = 8
for 2º Amine, (- NH -) C4H9 - NH - CH3 ⇒ 4 forms
Also, C3H7 - NH - C2H5
(2 form)
total form at 2º = 4 + 2 = 6
for 3º 
C3H7 -  (2 form)
(2 form)
Also,  (1 form)
(1 form)
Total form of 3º = 2 + 1 = 3
Total no. of amines = 8 + 6 + 3 = 17
Ex.13 For molecular formula C4H9NO, how many amides will be there that will not form H-bond?(Only structural)
Sol. (1º amide)
(1º amide)  (2º amide)
(2º amide)  (3º amide)
(3º amide)
for 1º amide
 (2 form)
(2 form)
for 2º amide,  (2 form)
(2 form) 

total 2º amide = 4
for 3º amide


3º amide will not form H-bond hence there will be 2 amides which will not form H-bond.
Ex.14 Find all 1º, 2º and 3º amides for C3H7NO(Only structural)
Sol. For 1º amides
 (1 form) , total 1º amide = 1
(1 form) , total 1º amide = 1
for 2º amide

 = 2 form
= 2 form
for 3º amide
 total 3º amide = 1
total 3º amide = 1
total amides (1º 2º 3º) = 1 + 2 + 1 = 4
Ex.15 Find the total no. of acid and esters from C4H8O2(Only structural)
Sol. For acid, (- COOH) C3H7 - COOH (2 form)
for ester,  → alcohol part
→ alcohol part

CH3COOH C2H5OH
for ester,  (2 form)
(2 form) 

total esters = 4
Ex.16 C4H4O4 may be (Only structural)
(i) Saturated dicarboxylic acid (ii) Unsaturated dicarboxylic acid
(iii) Cyclic diester (iv) Saturated di aldehyde
Sol. Three unsaturation.
(ii) and (iii) is the Ans.
 and
and 
Ex.17 How many aromatic isomers will be possible for C7H8O(Only structural)
Sol.





Total = 5
Ex.18 Find the possible dichloro derivatives of C6H4Cl2 (Only structural)
Sol.



Ex.19 C6H4Cl2 →C6H3Cl3 (Only structural)
 →
→
 →
→
 →
→
find the value of x, y, z.
Sol.

(1, 2, 4)
Two possibilities are there for placing Cl in place of H.


y = 3 There is one possibility of placing Cl, therefore z = 1
x = 2, y = 3, z = 1
Ex.20 Find the total carbonyl compound (aldehydes and ketones) formed by C5H10O and also find the relation between carbonyl compounds that have the same no. of a-hydrogen.(Only structural)
Sol. For ketones,
 and
and 
Isomerism -
(2 form)
total ketones = 3
for aldehydes 
(4 forms)
total aldehydes = 4
total carbonyl compounds = 4 + 3 = 7
CH3 - CH2 - CH2 - CH2 - CHO (2 α H)  (one α H)
(one α H)
 (2 α H)
(2 α H)  (no α hydrogen)
(no α hydrogen)
ketone,  (5 α H)
(5 α H)

Ex.21 Find total acyclic structural isomer of C6H12 (Only structural)
Sol.
(1)  = 3
= 3
(2)  = 4
= 4
(3)  = 1
= 1
(4)  = 2
= 2
(5)  = 3
= 3
total = 13 isomers.
Ex.22 Find the total conjugated diene in C5H8 (Only structural)
 →one possibility
→one possibility
 →one possibility
→one possibility
 no possibility of placing a double bond the valency of C will be more than four.
no possibility of placing a double bond the valency of C will be more than four.
Ex.23 Find the total cumulated diene in C5H8. (Only structural)
Sol.

 →
→
 →no form cumulated diene
→no form cumulated diene
total = 3
⇒ Isolated dienes,
C - C - C - C - C →
 →no form isolated diene
→no form isolated diene
total = 1
Metamerism
- Compounds having the same molecular formula but differ from the nature of the alkyl group directly attached to a polyvalent atom or polyvalent functional group.
- This type of isomerism is found in those types of compounds that have polyvalent atoms or polyvalent functional groups, e.g. ether, 2º amine, ester, etc.

Examples of metamers:
1.
2.
(a)
(b) CH3 - CH2 - CH2 - NH - CH2 - CH3
a & b are metamers.
Tautomerism
Compounds having the same molecular formula but different due to the oscillation of an atom (usually H ) are known as tautomers.



as after removal of H+, the anion formed is resonance stabilized.
Keto-enol Tautomerism:
 Keto-Enol Tautomerism
Keto-Enol Tautomerism
Mechanism :


*OH- acts as a catalyst.
Base Catalysed Tautomerism :



Mechanism:
* enol is more acidic than keto.
* After removal of H+ from both form.
 →
→ .......(i)
.......(i)
 →
→ .......(ii)
.......(ii)
In Ist -ve charge is on C and in IInd -ve charge is on O therefore (ii) is more stable than (i) hence enol form is more acidic than the keto form.
Acid Catalysed Tautomerism:



Ex.24




- In the case of base-catalyzed tautomerism, the stability of carbanion is the deciding factor.
- For acid-catalyzed tautomerism, the stability of the product will be the deciding factor.
Ex.25

 P1 + P2
P1 + P2
Sol.


 +
+ 
 (major) (minor)
(major) (minor)
(3 α H) +
+  (7 α H)
(7 α H)
(minor) (major)
Ex.26 Write the enol form:

Sol.

Generally keto form is more stable than enol but in some cases, the stability of enol form is greater than keto. This is due to
(i) Intramolecular H-bonding
(ii) Aromatic character
(iii) Extended conjugation
(iv) Steric factor
e.g. 
* Due to intramolecular H-bonding formation of 6-member ring takes place which is the cause of stability.
* This can be also summarized as:

If G = H % of enol 80 - 90%
G = Ph % of enol 90 - 99%
G = CH3 % of enol 70 - 80%
G = OC2H5 % of enol 5 - 10%
(one side)
* 
* Cross conjugation restricts the Resonance.

Ex.27 Compare the enol percent.
(A) CH3CHO
(B) 
(C) 
(D) 
(E) 
(F) 
(G) 
Sol. g > f > e > d > c > b > a
* In the case of a and b
After forming the enol form
CH2 = CH - OH (no ∝ H)
 more stable = higher %
more stable = higher %
* 
* 
*  enol % > keto %
enol % > keto %
*  (Keto < enol due to Intramolecular H-bond)
(Keto < enol due to Intramolecular H-bond)
* 
keto > enol
Ex.28 Find the enol form of the given compound.

Sol.

* The above case is called para tautomerism. Here g-Hydrogen participate in tautomerism.
Ex.29 Compare the enol content
(A) 
(B) 
(C) 
(D) 
Sol. After removing H (acidic-H) from the compounds
(A) 
(B) 
(C) 
(D) 
* Enol percent ∝ stability of carbanion
a > b > d > c
* Formation of carbanion is one of the steps from keto to enol. Therefore can be calculated as a percent of the stability of the carbanion.
Ex.30 Find the enol form of

Nitro and Acinitro form
 →
→


(acinitro)


 (acinitro)
(acinitro)

* (CH3)3 C - NO2 will not show nitro and acinitro form it has no H w.r.t to the NO2 group.
Imine and Enamine

For this type of tautomerism, there must a H w.r.t. (-CH = NH) group.
Amide and Amidol



Nitroso and Oxime form

II > I (stability)
due to extended conjugation in (II)
Hydrazone and Azoform
NH2 NH2 (Hydrazine)


CH3 - CH = N - NH - Ph  CH3 - CH2N = N - Ph
CH3 - CH2N = N - Ph
(extended conjugation)
Azo > Hydrazone (stability)
Ex.31 Compare enol percent.
(i) 
(ii) 
(iii) 
Sol. As we know C - D > C - H (Bond strength)
⇒ C - D will not break easily.
⇒ Compound will have less tendency to come into in enol form as C - D bond breaking is one of the step for conversion of keto into end.
⇒ enol percent will be less.
⇒ (i) > (ii) > (iii) (enol content)
Deuterium Exchange Reaction
(Deuterium Exchange Tautomerism)
 →
→







 →
→

 (final product)
(final product)
* To get the product directory replace all a-hydrogen w.r.t. carbonyl group by D(Deuterium):
e.g. 


Ring-chain Isomerism
 Ring Chain IsomerismIf one isomer has an open chain structure and the other has cyclic structure then isomers are known as ring-chain isomers and isomerism between them is known as ring-chain isomerism.
Ring Chain IsomerismIf one isomer has an open chain structure and the other has cyclic structure then isomers are known as ring-chain isomers and isomerism between them is known as ring-chain isomerism.
For examples :
(i) Alkene and cycloalkane, (CnH2n)
CH3 - CH = CH2
(ii) Alkyne and cycloalkene, (CnH2n - 2)
C4H6 : CH3 - CH2 - C º CH 
(iii) Alkenols and cyclic ethers, (CnH2nO)
C3H6O : CH2 = CH - CH2OH 
Note: Ring-chain isomers are always functional isomers.
|
127 videos|244 docs|87 tests
|
FAQs on Structural Isomerism - Chemistry Class 11 - NEET
| 1. What is isomerism? |  |
| 2. What is structural isomerism? |  |
| 3. What is chain isomerism? |  |
| 4. What is position isomerism? |  |
| 5. What is functional group isomerism? |  |

|
Explore Courses for NEET exam
|

|



















