The Hofmann and Curtius Rearrangements | Chemistry Optional Notes for UPSC PDF Download
Introduction
- Here’s the thing about the coverage of “amines” in Org 2: there’s no narrative.
- Unlike most chapters, it doesn’t start with a set of concepts and then build up to a series of examples you can then apply those concepts to.
- No. A typical chapter on amines in an introductory textbook is essentially just a hodge-podge of seemingly random topics thrown together that didn’t fit anywhere else. The only unifying thread is that they contain nitrogen in some way.
- Case in point: today’s random amine post is about the Hofmann and Curtius rearrangements.
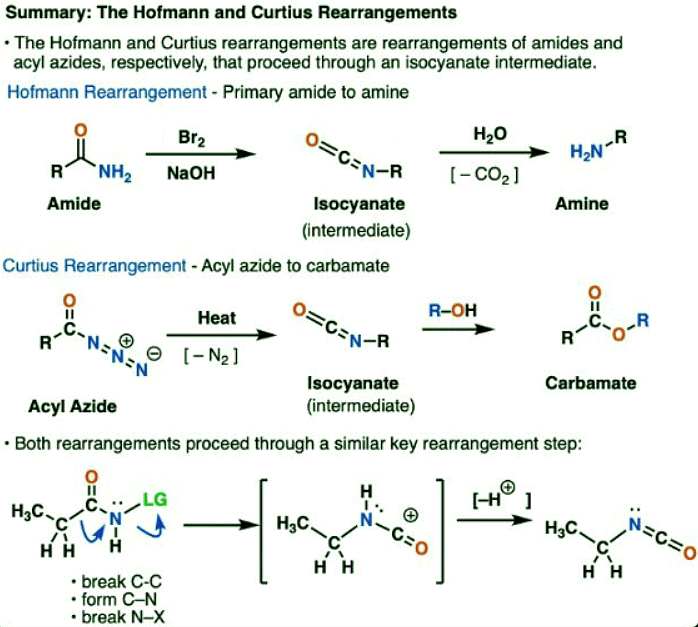
The Hofmann and Curtius Rearrangements
- The Hofmann and Curtius rearrangements are two examples of a whole family of rearrangement reactions that share a common mechanistic step.
- In the Hofmann rearrangement, an amide is treated with bromine and base (usually NaOH or KOH). Upon heating, an intermediate isocyanate is formed, which is not isolated. In the presence of water, the isocyanate loses carbon dioxide (“decarboxylates”) to give an amine.
- The key bond that forms in the Hofmann is the C2–N bond. Note how the carbonyl group (C1) is lost, forming carbon dioxide (CO2).

- In the Curtius rearrangement, an acyl azide is heated, and an isocyanate is formed. In the Curtius, the isocyanate can be isolated, but is usually transformed further into other species such a carbamate, a urea, or (via decarboxylation, as in the Hofmann) to an amine (more on those below).

- The key observation to note in both cases is that the C1-C2 bond has broken, and a new C2-N bond has formed.
The Mechanism of the Hofmann and Curtius Rearrangements, Part 1: Setting Up The Rearrangement
There are four important parts to the mechanism of the Curtius and Hofmann rearrangements, and we’re going to walk through them all.
- Prelude (straightforward)
- The key migration step (tricky, but less tricky if you realize you’ve seen a variant of it in Org 1 !)
- Formation of the isocyanate (straightforward)
[interlude: steps 2 and 3 actually happen at the same time, so we’ll show how to put them together] - Epilogue: Transformations of the isocyanate
Part 1: Prelude
- The Hofmann rearrangement occurs with an amide.
- The Curtius rearrangement occurs with an acyl azide.
Both are conveniently prepared from acyl halides through an addition-elimination reaction. If you’re covering amines now, then carbonyl reactions are likely familiar territory. The acyl halides can be prepared from carboxylic acids through using a reagent like thionyl chloride (SOCl2) or phosphorus pentachloride (PCl5).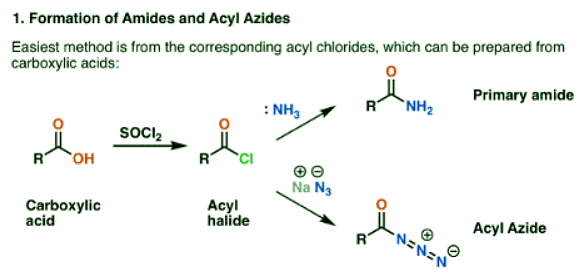
Setting up the Hofmann
In the Hofmann rearrangment, the amide is treated with bromine (Br2) and a base such as NaOH. This results in the breakage of N-H and the formation of N-Br, resulting in the installation of a good leaving group on the nitrogen. We call this an “N-bromo amide”.
The Curtius needs no “setting up”, as the acyl azide already has a splendid built-in leaving group: N2. This is why organic azides should be treated with care, as rough treatment can lead to explosions.
The Key Rearrangement Step In The Hofmann and Curtius
- Now we get down to business.
- The key step in the Hofmann and Curtius rearrangments is migration of a carbon atom to displace a leaving group on an adjacent nitrogen.
- This requires two curved arrows to draw, which are shown in the structure on the far left (below).
- In the first curved arrow, the C-C bond breaks, and a new C-N bond forms.
- In the second curved arrow, the N–LG (leaving group) bond breaks.
- However, it isn’t always easy to go directly from the structure on the far left to the structure on the far right (which is drawn nice and tidy! ).
- Countless points are needlessly lost in exams every year by students who get the curved arrows right but end up drawing the wrong structure! That’s why I encourage students to draw an “ugly version” first, which looks like crap but at least has all the bonds in the right place. There’s always time to redraw it and make it look pretty later.

- At first this rearrangement probably looks… odd. But it’s actually a reaction you’ve seen before! (but in disguised form).
- Anyone remember the mechanism for the “oxidation” step in hydroboration-oxidation?
- It’s basically the same thing!
- Here’s a refresher:

- Just another example of how things you learn in an earlier part of organic chemistry can come back in a later part of the course!
- Let’s visit the specific rearrangement step in the Hofmann and Curtius rearrangements with concrete examples.
- Here’s the key step in the Hofmann, where heating results in breakage of C-C , formation of C-N, and breakage of N-Br.

- If you follow all the arrows, you should end up with a weird-looking carbocation (above right) which we’ll deal with in a minute.
- In the Curtius, heating the acyl azide results in rearrangement. The leaving group is nitrogen gas (N2).

- (A subtle difference between the Curtius and the Hofmann is that there is no hydrogen attached to N in the Curtius, so we end up with a negative charge on the nitrogen in the rearranged species.)
Formation of the Isocyanate
- The next step is going from our rearranged species to the isocyanate. For anyone accustomed to drawing resonance forms, this shouldn’t be too difficult.
- (As we’ll discuss below, studies have determined that isocyanate formation occurs at the same time as migration. But for our teaching purposes, I think it helps to treat this step in isolation. We can put it all together in a minute).
- As you might recall from waayyyy back in Org 1, a resonance form with full octets is superior to one without (quick review). Our weird-looking product from rearrangement has a carbocation adjacent to a nitrogen containing a lone pair. So the first step is to draw the formation of a new C-N pi bond. This is essentially just a resonance form.
- The Hofmann rearrangement occurs in the presence of base. So after drawing the resonance form, the next step is deprotonation of the N–H bond giving the neutral isocyanate.

- There’s no hydrogen on the nitrogen in the Curtius. So isocyanate formation is achieved merely by donation of a lone pair from nitrogen to the carbocation. You can just look at it as drawing a resonance form.

Putting The Steps Together
- Studies into the mechanism of the Hofmann and Curtius rearrangements have determined that these two steps do not happen sequentially; they actually happen at the same time! (how do we know this? see Note 1 )
- That is, the migration happens at the same time as isocyanate formation.
- We need to redraw the mechanisms of the Hofmann and Curtius to reflect that, incorporating both steps.
- Here’s the Hofmann (note that the deprotonation event is actually separate).

- And the Curtius:

The Fate Of The Isocyanate
- Both the Hofmann and Curtius give rise to an isocyanate. You likely haven’t encountered isocyanates before. At first glance, they are pretty strange-looking species, but their chemistry is not so different from that of other carbonyl species such as esters, amides, and acyl halides.
- The isocyanate structure closely resembles that of carbon dioxide, CO2. The carbon is pi-bonded to two strongly electronegative atoms. This makes the carbon an excellent electrophile.
- By adding various nucleophiles, isocyanates can be transformed into other useful species.
- Adding an alcohol results in formation of a carbamate
- Adding an amine results in a urea
- Adding water results in a carbamic acid, which is unstable. Carbamic acids quickly lose carbon dioxide to give an amine.

- In particular, the decarboxylation pathway presents a nifty way to make amines. We’ve learned tons of ways to make carboxylic acids, but not too many ways of making amines (besides nucleophilic substitution and reductive amination). The Hofmann is a good trick to have in your back pocket in situations where neither of those situations might apply – like making substituted anilines, for example.
Notes
Note 1: Concerted or separate steps?
If the Curtius was stepwise, the loss of nitrogen would lead to a nitrene. Nitrenes undergo a host of interesting reactions, such as insertion into C-H bonds (!). So one can design an experiment to test this: if one tries the following Curtius rearrangement, one could look for evidence of formation of a five-membered ring.
The absence of such products, along with other evidence, points to a concerted mechanism.
Note 2: Isocyanates
The most important mechanism of isocyanates is electrophilic addition (i.e. adding a nucleophile to the central, electrophilic carbon). In the mechanism below I showed the nucleophile adding to the carbon so as to form an anion on the nitrogen, although this is is likely not the best resonance form (the oxygen is better at stabilizing negative charge than is nitrogen). Proton transfer from oxygen to nitrogen results in a neutral species. (I used the Magic Wand of Proton Transfer here, because it’s faster).
Note that the decarboxylation step in 1) doesn’t need to be preceded by deportation of the oxygen, although it definitely needs to occur such that nitrogen is protonated during the step (you don’t want to form a strongly basic amide ion, for instance).
Note 3: The Beckmann, Wolff, and other rearrangements share a similar step
- But wait, there’s more!
- There’s a 1,2-shift with loss of a leaving group in the Beckmann rearrangement. The key step is essentially the same as we saw in the Hofmann and Curtius.

- And in the Wolff rearrangement, which is commonly also covered, you might also recognize the same key step.

- And that’s not all! There’s a reaction called the Schmidt rearrangement, which is like the Curtius, but comes from adding HN3 to an acyl chloride (or carboxylic acid). And there’s another one called the Lossen rearrangement that occurs with hydroxyamic acids. No prizes for guessing the key step in those two processes. And we haven’t even mentioned the Baeyer-Villiger, which doesn’t involve nitrogen, but is pretty much the same type of process.
- When I learned the hydroboration-oxidation step in Org 1, many moons ago, I certainly never expected that I would see the same reaction pattern repeat itself in so many different guises.
- The bottom line is that there are a lot of reactions that go by different names that proceed through the same mechanistic process. This highlights the importance of learning the key patterns in organic chemistry, because they sure do repeat themselves a lot.
Summary: The Hofmann and Curtius Rearrangements
The mechanism of these rearrangments is tricky at first glance, but is made considerably easier once you realize that you’ve seen a variant of the migration reaction before (i.e. in hydroboration-oxidation).
I highly recommend drawing the ugly version first, because it will help you focus on seeing the bonds that form and break, before trying to redraw it into a more aesthetically appealing structure. This also goes for the Beckmann and Wolff rearrangements, which have a similar step.
FAQs on The Hofmann and Curtius Rearrangements - Chemistry Optional Notes for UPSC
| 1. What are the Hofmann and Curtius rearrangements? |  |
| 2. What is the key rearrangement step in the Hofmann and Curtius rearrangements? |  |
| 3. How is the isocyanate formed in the Hofmann and Curtius rearrangements? |  |
| 4. What happens to the isocyanate in the Hofmann and Curtius rearrangements? |  |
| 5. What is the significance of the Hofmann and Curtius rearrangements in synthetic chemistry? |  |

|
Explore Courses for UPSC exam
|

|























