Class 10 Exam > Class 10 Notes > Physics for GCSE/IGCSE > The Nucleus
The Nucleus | Physics for GCSE/IGCSE - Class 10 PDF Download
| Table of contents |

|
| Composition of the Nucleus |

|
| Describing the Nucleus |

|
| Nuclide Notation |

|
| Isotopes |

|
Composition of the Nucleus
- The nucleus consists of a positively charged center made up of protons and neutrons, surrounded by negatively charged electrons orbiting around it.
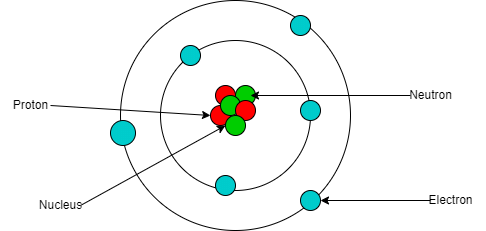
- Protons carry a positive charge, while neutrons possess no charge.
- Hence, the nucleus exhibits an overall positive charge.
Describing the Nucleus
Proton Number, Z
- The number of protons in an atom is known as its proton number, which is also called the atomic number.
- Elements in the periodic table are arranged based on their atomic number, determining the identity of an element.
- For instance:
- Hydrogen has an atomic number of 1, indicating it has one proton.
- Sodium has an atomic number of 11, corresponding to 11 protons.
- Uranium has an atomic number of 92, representing 92 protons.
- The atomic number is equal to the number of electrons in an atom, ensuring overall charge neutrality.
Nucleon Number, A
- Number of Neutrons: The number of neutrons in an atom can be calculated by subtracting the atomic number (number of protons) from the mass number (nucleon number).
- For instance, consider a sodium atom with a mass number of 23 and an atomic number of 11. Therefore, the number of neutrons would be 23 - 11 = 12.
Question for The NucleusTry yourself: What is the atomic number of an element that has 10 protons?View Solution
Nuclide Notation
- A nuclide refers to a cluster of atoms that possess an identical count of protons and neutrons. For instance, if there are 5 atoms of oxygen, they all constitute the same nuclide, even though they exist as separate entities.
- Atomic symbols are denoted using a specific notation known as nuclide or ZXA notation.
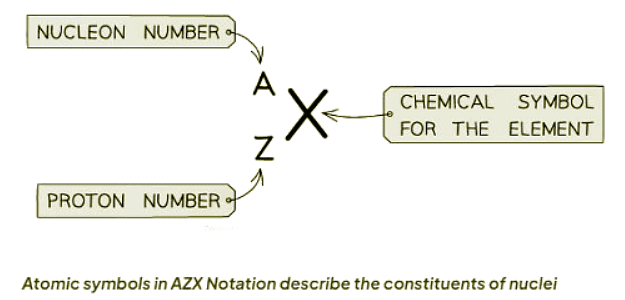
- The top number denoted as A signifies the nucleon number or mass number, representing the total count of protons and neutrons within the nucleus.
- The lower number denoted as Z signifies the proton or atomic number, indicating the total count of protons within the nucleus.
- In Chemistry, the nucleon number corresponds to the mass number, and the proton number corresponds to the atomic number. The periodic table is arranged by atomic number.
- Example of an Atomic Symbol: An atomic symbol, such as the one shown in the image below, provides information about the components of atomic nuclei.
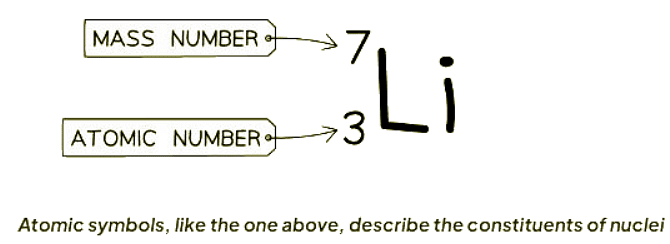
- When you are presented with an atomic symbol, it is possible to determine the total number of protons, neutrons, and electrons in the atom:
- Protons: Protons are equivalent to the proton number of the atom.
- Electrons: In a neutral atom, the number of electrons, which are negatively charged, matches the number of protons, which are positively charged.
- Neutrons: Neutrons can be calculated by subtracting the proton number from the nucleon number.
The Concept of Nucleons and Nuclides:
- Nucleons: Nucleons refer to particles found in the nucleus, such as protons or neutrons.
- Nuclides: A nuclide is a nucleus characterized by a specific combination of protons and neutrons.
Isotopes
- Isotopes are atoms of the same element having the same number of protons but varying numbers of neutrons. This disparity in neutron count leads to different isotopes within an element.
- Each element can exhibit multiple isotopes due to the presence of varying neutron numbers alongside a constant proton count.
- Isotopes, characterized by their neutron-proton imbalances, are generally less stable than elements with balanced neutron-proton ratios. Consequently, they possess a higher likelihood of undergoing decay processes.
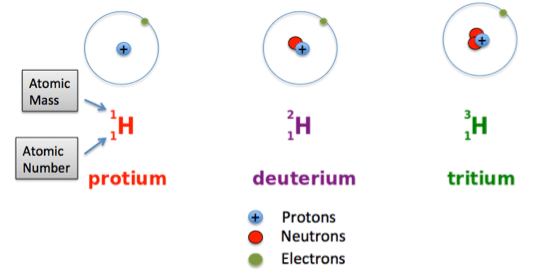
- Isotopes are naturally-occurring, but their abundance varies.
- For instance, approximately 2 out of every 10,000 hydrogen atoms are Deuterium.
- Tritium is even scarcer, with only about 1 in every billion billion hydrogen atoms being Tritium.
The document The Nucleus | Physics for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Physics for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
126 videos|194 docs|35 tests
|
FAQs on The Nucleus - Physics for GCSE/IGCSE - Class 10
| 1. What is the composition of the nucleus? |  |
Ans. The nucleus is composed of protons and neutrons which are held together by strong nuclear forces.
| 2. How is the nucleus described in terms of atomic structure? |  |
Ans. The nucleus is the central part of an atom that contains the majority of the atom's mass and is positively charged due to the presence of protons.
| 3. What is nuclide notation and how is it used to represent the nucleus? |  |
Ans. Nuclide notation is a way of representing a specific isotope of an element by indicating the atomic number, mass number, and the chemical symbol of the element. It helps to distinguish between different isotopes of the same element.
| 4. What are isotopes and how do they relate to the nucleus? |  |
Ans. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. They differ in their atomic mass but have the same chemical properties. Isotopes are related to the nucleus as they represent different configurations of protons and neutrons within the nucleus.
| 5. How does the nucleus play a crucial role in the overall structure of an atom? |  |
Ans. The nucleus is the central core of an atom that contains the majority of the atom's mass and positive charge. It houses the protons and neutrons, which determine the element's identity and properties. The nucleus plays a crucial role in determining the stability and behavior of an atom.
Related Searches



















