The Wolff-Kishner, Clemmensen, And Other Carbonyl Reductions | Chemistry Optional Notes for UPSC PDF Download
Introduction
In this post we go through 4 ways of reducing C=O to CH2 including:
- The Wolff-Kishner reaction
- The Clemmensen reduction
- Catalytic hydrogenation
- Thioacetal formation and reduction
But first: why might you want to do this? Well, it’s a key component in a common little synthesis problem I like to call “The Great Friedel-Crafts Workaround”.
The Great Friedel-Crafts Workaround
- A recent post covered oxidations on the “benzylic” carbon (i.e. on the carbon adjacent to the aromatic ring). We showed that benzylic C-H bonds are unusually weak, and can be converted relatively easily (and selectively!) to C–Br or C–O bonds.
- Today we’re going to go in the reverse direction and address reduction of the benzylic carbon, notably reduction of ketones (C=O) to alkyl (CH2).
- This is particularly important because of the Great Friedel-Crafts Workaround.
- What’s that, you ask?
- You may recall that Friedel-Crafts alkylation of aromatic rings with primary alkyl halides can result in carbocation rearrangements. For example, attempting a Friedel-Crafts alkylation of benzene with propyl chloride results in isopropylbenzene, not propyl benzene.

- The Great Friedel-Crafts Workaround solves this issue. We begin with a Friedel-Crafts acylation, which proceeds without rearrangement, and follow by reducing the ketone down to CH2.
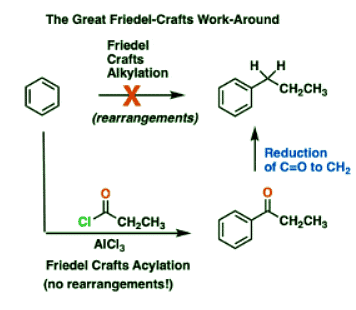
- So how can we reduce the ketone down to an alkane? Four ways.
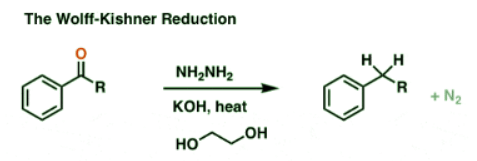
The Wolff-Kishner Reduction Of Ketones
- The Wolff Kishner reduction of ketones utilizes hydrazine (NH2NH2) as the reducing agent in the presence of strong base (KOH) in a high-boiling protic solvent (ethylene glycol, HO-CH2CH2-OH, boiling point 197 °C).
- The driving force for the reaction is the conversion of hydrazine to nitrogen gas.

- This is not exactly a gentle process; heating to almost 200 °C is required to make the reaction occur at a reasonable rate. [Note 1]
- The first step is formation of a hydrazone from the ketone (hydrazones are a cousin of imines, which we cover later in the course). Hydrazine (NH2NH2) adds to the carbonyl, and following a series of proton transfer steps, water is expelled.

- Once the hydrazone is formed, the real action in the Wolff-Kishner begins!
Mechanism Of The Wolff-Kishner Reaction
- The NH2 of the hydrazone is reasonably acidic (pKa about 21) and can be deprotonated by strong base at a high enough temperature (the base is likely the conjugate base of ethylene glycol, not KOH). This deprotonation appears to be the rate-limiting step.
- The next step is the trickiest: protonation on the carbon. With the caveat that resonance forms don’t really exist, it can be helpful to imagine forming the resonance form of this species that has a negative charge on the carbon, and then protonating it with solvent (ethylene glycol).
- This gives a species with a nitrogen-nitrogen double bond, which, after deprotonation by base, decomposes irreversibly to give nitrogen gas and a carbanion (i.e. a negatively charged carbon).
- Protonation of the carbon completes the process.

The Clemmensen Reduction of Ketones
- A second way to go about reducing the carbonyl of an aromatic ketone is to use a reaction known as the Clemmensen Reduction. The reductant here is “zinc amalgam” (Zn-Hg) which is used under acidic conditions; one method calls for the presence of aqueous HCl, for example:

- This process works best for aromatic ketones; non-aromatic ketones, not so much. The mechanism has not been thoroughly worked out; it’s thought to occur through a series of one-electron transfers from zinc amalgam.
Why Would You Prefer The Wolff-Kishner Over the Clemmensen, Or Vice Versa?
It’s somewhat rare to encounter conditions in an introductory class where a Wolff Kishner would be called for over a Clemmensen, or vice versa, but here are some things to think about.
- The Wolff-Kishner is done under strongly basic conditions using high heat in a polar protic solvent.
- The Clemmensen is performed in strongly acidic conditions. If you have a protecting group somewhere which can be removed with acid, such as an acetal or silyl ether, consider an alternative.
Two other methods deserve mention, although you might not seen them covered until later in the course when ketone chemistry is addressed.
A Third Method For Carbonyl Reduction: Catalytic Hydrogenation
- The first is catalytic hydrogenation, i.e. using a metal catalyst such as Pd/C or Pt/C with hydrogen gas (H2).
- We’ve mostly seen catalytic hydrogenation used for reducing alkenes and alkynes, but it can also be used for ketones if you crank on it enough (i.e. higher temperatures, with higher pressure of H2).
- One subtle tweak in conditions, sometimes not mentioned, is that platinum on carbon (Pt-C) or platinum oxide (PtO2) is often used instead of palladium (Pd).
- Normally, reduction of ketones usually stops at the alcohol stage. However, in the case where the alcohol is on a benzylic position, (i.e. on a carbon adjacent to an aromatic ring) reduction with can occur further to the alkane (recall that bonds at benzylic positions tend to be easier to break, since the adjacent aromatic ring can donate electron density to them).

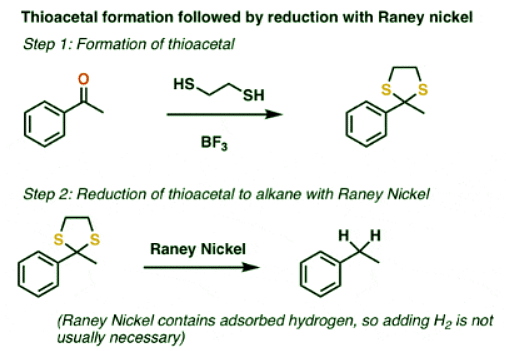
Reduction of Thioacetals
- The second method that sees use is conversion of the ketone to a “thioacetal” with HS-CH2CH2-SH and a Lewis acid such as BF3. [Note 2] This is followed by treating the thioacetal with a reducing agent known as Raney Nickel: it’s a form of finely divided nickel containing adsorbed hydrogen that cleaves C-S bonds to give C-H bonds, through a somewhat mysterious process also thought to involve free-radicals.

- Unlike the Clemmensen and catalytic hydrogenation, thioacetal/Raney nickel method isn’t limited merely to benzylic ketones. It can be used to completely “disappear” an aldehyde or ketone, as was part of the strategy in Woodward’s synthesis of erythromycin.
A Workaround Example
With these methods in our toolbox, we can now fill in the vague description “reduction” over the arrow with something a lot more specific.
Here’s a concrete example of a Friedel-Crafts Workaround:
Notes
- Note 1: One way of getting around the requirement for high heat in the Wolff-Kishner is to use a strong base like t-BuOK in DMSO, which can be done around room temperature.
- Note 2: Lewis acids such as BF3 or ZnCl2 are commonly used for this reaction although in practice, protic acids such as HCl are perfectly fine if the starting material is an aldehyde or ketone.
In Corey and Seebach’s method for making 1,3-dithiane, the starting materials are 1,3-propanedithiol and dimethoxymethane (CH3OCH2OCH3), an equivalent of formaldehyde. BF3, a strong Lewis acid, gave better yields in this procedure than did anhydrous acid, subsequently BF3 seems to be the Lewis acid of choice in organic chemistry textbooks despite the fact that most thioacetal formation reactions seen in introductory courses are from aldehydes or ketones, not acetals.
A Final Note: Reversing Polarity
A final thing to note here is that reduction of a benzylic ketone to the alkane reverses the polarity of the substituent. It converts an electron-withdrawing meta-director (an acyl group) into an electron-donating ortho-, para- director. We’ll have more to say about this when we address synthesis in aromatic compounds, but just take a gander at these two examples…. Two more examples of reversing the polarity of a substituent is reduction of the nitro group and Baeyer-Villiger oxidation of a ketone to an ester.
Two more examples of reversing the polarity of a substituent is reduction of the nitro group and Baeyer-Villiger oxidation of a ketone to an ester.
FAQs on The Wolff-Kishner, Clemmensen, And Other Carbonyl Reductions - Chemistry Optional Notes for UPSC
| 1. What is the purpose of the Great Friedel-Crafts Workaround? |  |
| 2. What is the mechanism of the Wolff-Kishner reaction? |  |
| 3. How does the Clemmensen reduction of ketones differ from the Wolff-Kishner reaction? |  |
| 4. When would you prefer the Wolff-Kishner over the Clemmensen reduction, or vice versa? |  |
| 5. What is catalytic hydrogenation and how is it used for carbonyl reduction? |  |

|
Explore Courses for UPSC exam
|

|