Theory & Procedure, Determine pH with pH indicator strips / universal indicator solution | Science Class 10 PDF Download
Our Objective
To find the pH of the following samples by using pH paper / universal indicator.
- Dilute hydrochloric acid.
- Dilute NaOH solution.
- Dilute ethanoic acid solution.
- Lemon juice.
- Water.
- Dilute sodium bicarbonate solution.
The Theory
Can you define pH?
pH is defined as the negative logarithm(base 10) of the hydrogen ion concentration in moles per litre. pH is written as:
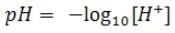
Hydrogen ions cannot exist alone, but they exist after combining with water molecules. Thus, H+must always be shown as H+(aq) or hydronium ion(H3O+):
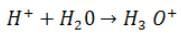
How do we represent the pH of a neutral solution?
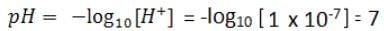
Neutral solution has pH value 7. pH of acid solution is always less than 7, whereas that of alkaline solution is always more than 7. pH of pure water is 7 or [H+]=10-7 mol/L .
What is a pH indicator?
pH indicator is a chemical that turns different colours in different media. For example, blue litmus turns red in acidic medium, the red litmus turns blue in alkaline medium.
What is a litmus solution?
Litmus solution is a purple dye, which is extracted from a Thallophyte known as Lichen. Litmus solution when in neutral medium (i.e., neither acidic nor basic) is greenish in colour.
What are the commonly used indicators to determine pH of a solution?
Litmus solution and litmus paper are commonly used as an indicator. pH paper is a piece or a strip of paper which is coated with pH-indicator.
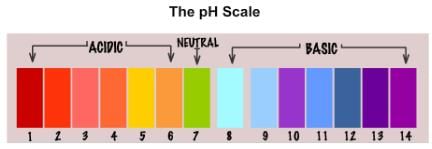
Know what a pH scale is?
A scale for measuring hydrogen ion concentration is known as pH scale. In the term pH, p stands for 'potenz' in German meaning power.
Learning Outcomes
- Students understand the terms pH, acidic, basic and neutral through the experiments.
- Students acquire the following skills after performing the experiment.
- How to measure the pH of a given solution using pH paper or universal indicator solution.
- How to classify the sample as acidic, basic or neutral based on the pH value.
- How to correlate the values obtained from the experiment with the pH scale
Materials Required:
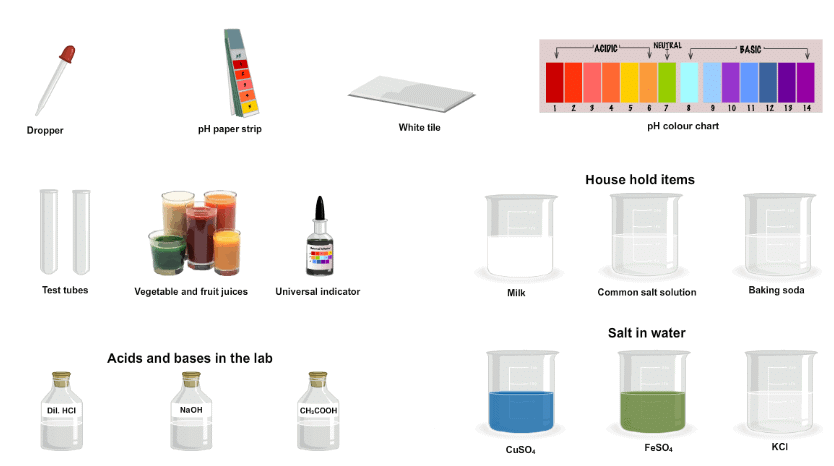
The Procedure:
As performed in a real lab:
- Take six strips pH paper and place them on a glazed tile. Mark them 1 to 6.
- Take the test solutions in separate test tubes. Dissolve the solid substance by adding distilled water to it. Label the test tubes.
- Now, place a drop of the test solution on one strip of the pH paper with the help of a fine dropper or glass rod. Use a fresh dropper for each test solution.
- Observe the colour produced and match it with the different colour shades of the standard colour pH chart.
- Note down the colour of the pH from the colour chart that matches most closely with the colour produced on the pH paper.
- Similarly, find the pH value of the remaining samples by using a fresh strip of pH paper and a separate glass rod or fine dropper for each one.
As performed using the simulator:
Using the pH strip:
Using the Universal Indicator:
- You can select the aqueous solution type you want to find the pH value of, from the ‘Select Aqueous Solution’ drop down list (vegetable & fruit juices, household items, acids and bases in the lab or salts in water).
- Now choose any one of the solutions in the beaker by clicking on it.
- There are two ways of finding the pH value of the solution:
- Click and drag the dropper from the stand and move into the solution in the beaker to collect the solution in the dropper.
- Still holding the dropper, move it from the beaker over to the pH strip and release it.
- To find the pH value of the solution, select the colour from the menu on the left by clicking and dragging it to the pH strip and comparing it.
- The colour that matches with the spot on the pH strip indicates the pH value of the solution.
- Click and drag the dropper from the universal indicator bottle and move it into the solution in the beaker to drop the universal indicator into it.
- To find the pH value of the solution, select the colour from the menu on the left by clicking and dragging it next to the solution in the beaker and comparing it.
- The colour that matches with the solution in the beaker indicates the pH value of the solution.
Note:
- Once test is done using the Universal Indicator, you cannot do it with the pH strip. To do it with the pH strip, click the ‘Reset’ button and vice versa.
- The ‘Reset’ button can be used to redo the experiment with other solutions.
Observations:
Sl.No | Sample solution | Colour produced on pH paper | Approximate pH | Inference |
1 | Dil.HCl | |||
2 | Dil. NaOH | |||
3 | Dil. Ethanoic acid | |||
4 | Lemon juice | |||
5 | Water | |||
6 | Dil. Sodium bicarbonate sol. |
Precautions:
- Use only the standard colour chart supplied with the pH paper for assessing the ph value.
- Keep the pH strips away from chemical fumes.
- Either use fresh fine dropper or glass rod for each different sample, or wash the dropper or rod well with water every time.
|
83 videos|438 docs|74 tests
|
FAQs on Theory & Procedure, Determine pH with pH indicator strips / universal indicator solution - Science Class 10
| 1. How do pH indicator strips work? |  |
| 2. What is the procedure for using pH indicator strips? |  |
| 3. What is universal indicator solution? |  |
| 4. How accurate are pH indicator strips in determining pH? |  |
| 5. Can pH indicator strips be used to determine the pH of any solution? |  |


















