Tropones And Tropolones | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Non-benzenoid Aromatics |

|
| Tropone and Tropolone |

|
| Synthesis |

|
| Reactions of Tropones and Tropolones |

|
Non-benzenoid Aromatics
Aromatic systems that are not comprised of benzene ring e.g.,


For a compound to be aromatic it must follow Hückel's rule : i.e.
- Molecule must be cyclic and planar
- Must have an fully conjugated cyclic π electron cloud.
- It must have (4n + 2) π electrons i.e. compounds containing 2, 6, 10, 14, 18, …..πelectrons
All three conditions should be fulfilled
For example, consider cycloheptatrienyl cation and anion-Both are cyclic, planar and exhibit continuous delocalization of π electrons , yet cycloheptatrienyl cation with 6π electrons is aromatic while cycloheptatrienyl anion with 8π electrons is not aromatic.

- Similarly, Cyclodentadiene anion is aromatic while, cyclopentadiene (lacks continuous delocalization and (4n + 2) π electrons , cyclopentadienyl radical and cyclopentadienyl cation (both lacking (4n + 2) π electrons) are not aromatic.

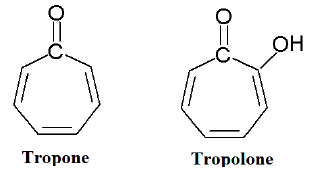
Tropone and Tropolone
Tropone (2,4,6-cycloheptatrien-1-one) and tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) are non-benzenoid aromatic compound.
- It was proposed by Dewar that tropones could have aromatic properties as the polarization of C=O group will lead to a partial positive charge (d+) on the carbon atom and a partial negative charge (d-) on oxygen (I).
- In some extreme cases complete polarization of C=O bond is also possible (II) forming a tropylium ion ring which is an aromatic 6 p-electron system (III).

- Similarly, tropolone IV will also be aromatic by virtue of contribution by structure V

- Dipole Moment
Dipole Moment of numerous troponoids have been measured. They generally show high dipole moment indicating contribution from the polarized form (VI)

- The C=O Stretching Vibration (IR spectrum)
These stretching frequencies are lower than the usual C=O vibration because of high degree of conjugation and high polarity. This again indicates the greater contribution of
structures like (VI).
- The C-OH Stretching Vibration Tropolone (IR Spectrum)

Dimeric in solid state froms intramolecular H-bonding Monomeric in liquid state forms intramolecular H-bonding
Monomeric in liquid state forms intramolecular H-bonding
1H NMR: Ring protons in tropone appear at approximately the same filed as that of benzene butthose of tropolone appear slightly lower field.
Occurrence
Tropones and tropolones are found in many natural products. Many of these can be isolated from essential oil of trees. e.g., Hinokitiol, (tropolone compound) exhibits antibacterial effect. Alkaloid
Colchicine (with a tropolone ring) shows strong antitumor effects.


Synthesis
- By selenium dioxide oxidation of cycloheptatriene

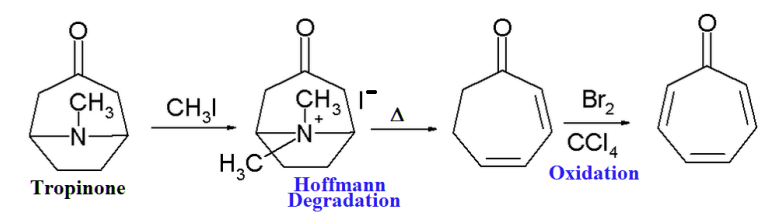
- From tropinone by following the reaction sequence shown below:

- By acyloin condensation of ethyl ester of pimelic acid followed by oxidation by Br2.

Reactions of Tropones and Tropolones
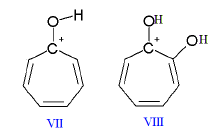
- In the presence of strong acids, tropone and tropolone form conjugate cations (VII and VIII, respectively)
- They also form picrates

- Electrophilic and free-radical substitution should occur at the 2-position for tropone.
- Bromination of tropone and its derivatives does go in the 2- position as predicted, but tropones first tend to form addition compounds and later give substitution products by dehydrohalogenation

- Nucleophilic reaction on tropone: The reaction of tropone with hydroxylamine yields a mixture of the oxime and 2-aminotropone in proportions depending on the reaction conditions.

- A large number of tropolone derivatives with free 5-position undergo azo-coupling, nitrosation (followed by reduction), nitration reaction etc. at 5-position as shown below:

FAQs on Tropones And Tropolones - Chemistry Optional Notes for UPSC
| 1. What are non-benzenoid aromatics? |  |
| 2. What are tropone and tropolone? |  |
| 3. How are tropone and tropolone synthesized? |  |
| 4. What are some reactions of tropones and tropolones? |  |
| 5. How do tropones and tropolones relate to the UPSC exam? |  |

|
Explore Courses for UPSC exam
|

|




 Monomeric in liquid state forms intramolecular H-bonding
Monomeric in liquid state forms intramolecular H-bonding