Wetting Agents | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Types of bulk intermolecular forces |

|
| Types of Wetting Agents |

|
| How to Tell if a Liquid Contains a Wetting Agent |

|
| Solved Examples |

|
Types of bulk intermolecular forces
There are two general types of bulk intermolecular forces:
- Cohesive Forces: The forces exerted between molecules holding them together. If cohesive forces are strong, a liquid tends to form droplets on a surface.
- Adhesive Forces: The forces between liquid molecules and a surface. If adhesive forces are strong, a liquid tends to spread across a surface.
Wetting agents are substances that reduce the surface tension of water to allow it to spread drops onto a surface, increasing the spreading abilities of a liquid. Lowering the surface tension lowers the energy required to spread drops onto a film, thus weakening the cohesive properties of the liquid and strengthening its adhesive properties. One example of how wetting agents work is in the formation of micelles. Micelles consist of hydrophilic heads forming an outer layer around lipophilic tails. When in water, the micelles' tails can surround an oil droplet while the heads are attracted to the water.

Dish soap is a great example of a wetting agent. With all the food oils and such on the plate cohesive forces make it difficult for the water to spread and clean the plate. The soap dissolves all theses unwanted particles, exposing a clean surface. The soap also lowers the surface tension of water, allowing it to spread evenly across the entire surface.
Types of Wetting Agents
- Anionic, cationic, and amphoteric wetting agents ionize when mixed with water.
- Anions have a negative charge, while cations have a positive charge.
- Amphoteric wetting agents can act as either anions or cations, depending on the acidity of the solution.
- Nonionic wetting agents do not ionize in water. A possible advantage for using a nonionic wetting agent is that it does not react with other ions in the water, which could lead to formation of a precipitate.
How to Tell if a Liquid Contains a Wetting Agent
- One method of knowing whether or not a liquid has a wetting agent in it is to spread the liquid on a surface that is coated in grease. If the liquid does not contain a wetting agent, the its cohesive forces would overpower adhesive forces, causing the liquid to for droplets on the surface (Figure 2, left). If the liquid does contain a wetting agent, the grease would be dissolved and the surface tension of the liquid would be lowered, causing the adhesive forces to overpower the cohesive forces. This would result in the liquid spreading evenly along the surface (Figure 2, right).

Figure 2: (left) A water droplet is sitting on a brass surface while being immersed in oil as an example of poor wetting. (right) A water droplet is sitting on a glass surface while being immersed in oil as an example of better wetting. Images used with permission from Wikipedia (Guro Aspenes, SINTEF Petroleum Research).
- Another method is to place the liquid in a test tube and observe the liquid's meniscus (Figure 3). If the liquid contains a wetting agent, its adhesive forces are stronger than cohesive forces, which means the liquid molecules are more inclined to stick to the surface than other liquid molecules. This results in a concave meniscus. If the liquid does not contain a wetting agent and is naturally very cohesive, like mercury, it forms a convex meniscus. This is caused by the fact the the molecules of the liquid have a stronger attraction to each other than to the surface of the test tube.
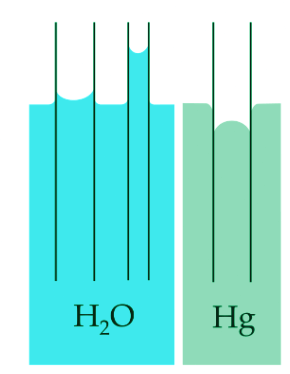
Intermolecular forces also cause a phenomenon called capillary action, which is the tendency of a polar liquid to rise against gravity into a small-diameter tube (a capillary), as shown in Figure 3. When a glass capillary is put into a dish of water, water is drawn up into the tube. The height to which the water rises depends on the diameter of the tube and the temperature of the water but not on the angle at which the tube enters the water. The smaller the diameter, the higher the liquid rises.
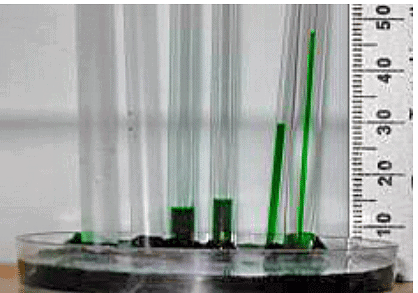
Figure 4: The Phenomenon of Capillary Action. Capillary action seen as water climbs to different levels in glass tubes of different diameters.
Solved Examples
Example 1: Would it be beneficial to use a wetting agent when waxing a car?
Ans: No, when waxing a car, you do not want water to wet the car's surface.
Example 2: An unknown liquid forms a convex meniscus when poured into a test tube. Does the liquid wet the test tube?
Ans: No, if it forms a convex meniscus, its cohesive forces overpower its adhesive forces, causing the liquid's molecules to want to stick to each other as much as possible.
Example 3: Do wetting agents increase or decrease the adhesive properties of a liquid?
Ans: They increase the adhesive properties of a liquid.
Example 4: Soap can form a precipitate when used as a wetting agent. Is it a nonionic or ionic wetting agent?
Ans: It must be an ionic wetting agent, since nonionic wetting agents do not form precipitates.
Example 5: A liquid's cohesive forces overwhelm its adhesive forces. Do you think it contains a wetting agent?
Ans: The liquid most likely does not contain a wetting agent, since it is more inclined to stick to itself than to wet the surface.
FAQs on Wetting Agents - Chemistry Optional Notes for UPSC
| 1. What are the different types of bulk intermolecular forces? |  |
| 2. What are some examples of wetting agents? |  |
| 3. How can you tell if a liquid contains a wetting agent? |  |
| 4. How do wetting agents work? |  |
| 5. What are the benefits of using wetting agents? |  |


















