Werner’s Theory & Some Basic Concepts of Coordination Compounds | Chemistry Class 12 - NEET PDF Download
What is Werner’s Theory?
Werner's Theory, proposed by Swiss chemist Alfred Werner in 1898, revolutionized the understanding of coordination compounds and laid the foundation for modern coordination chemistry.
Werner's Theory was a significant advancement in explaining the structure and bonding in complex compounds involving transition metal ions and ligands. The key aspects of Werner's Theory are as follows:
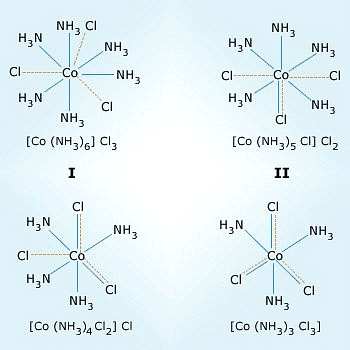 Dotted line represents the primary valency. Normal line represents the secondary valency.
Dotted line represents the primary valency. Normal line represents the secondary valency.
- Primary and Secondary Valence: Werner introduced the concept of primary and secondary valence to explain the behavior of transition metal ions in coordination compounds. The primary valence represented the oxidation state of the metal ion and determined its chemical reactivity. The secondary valence indicated the number of ligands coordinated to the metal ion.
- Coordination Sphere: According to Werner, a coordination compound consists of a central metal ion or atom surrounded by ligands. The coordination sphere is composed of the central metal ion and the ligands coordinated to it.
- Coordination Number: Werner proposed that the coordination number is the total number of ligands directly bonded to the central metal ion within the coordination sphere. The coordination number determines the geometry and stability of the complex.
- Isomerism in Coordination Compounds: Werner's Theory also explains the phenomenon of isomerism in coordination compounds. He identified two types of isomerism: geometric isomerism, where the spatial arrangement of ligands differs around the metal ion, and linkage isomerism, where the coordination bond occurs through different atoms of the ligand.
Werner's Theory provided a comprehensive framework for understanding the formation, structure, and properties of coordination compounds. His concepts of primary and secondary valence, coordination sphere, coordination number, and isomerism significantly advanced the field of coordination chemistry. Werner's contributions earned him the Nobel Prize in Chemistry in 1913, recognizing his pioneering work in the field of inorganic chemistry.
Werner’s Experiment
Alfred Werner made significant contributions to the understanding of coordination compounds with his theory and experiments conducted in 1898.
Remarkably, this theory was developed within a short span of just three years, from 1890 to 1893.
Following the formulation of his theory, Werner dedicated the rest of his career to gathering the necessary experimental evidence to support and validate his innovative ideas.
His pioneering work in the field of atomic linkage and coordination theory earned him the prestigious Nobel Prize in Chemistry in 1913, making him the first Swiss chemist to receive this honor.
Postulates of Werner’s Theory
Based on this observation, the following Werner’s theory was postulated:
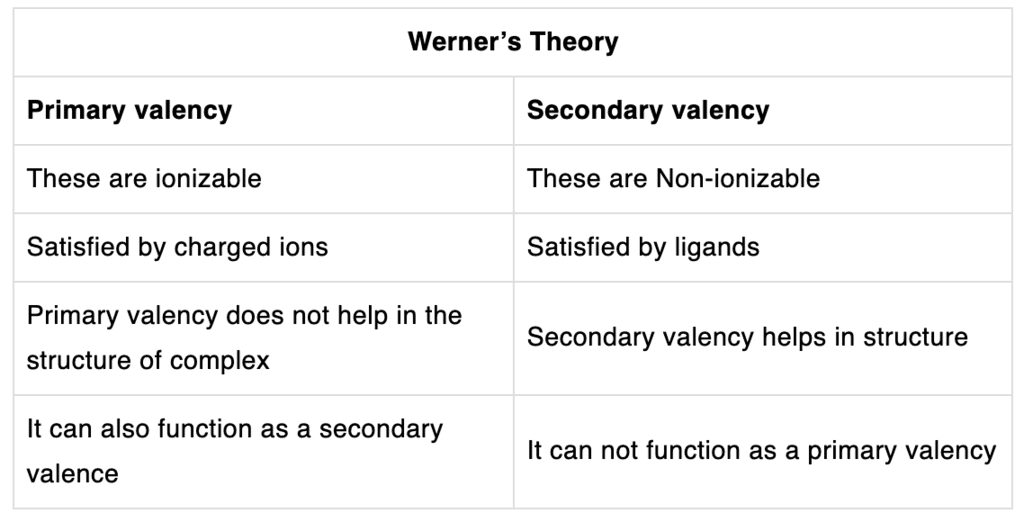
- The central metal atom in the coordination compound exhibits two types of valency, namely, primary and secondary linkages or valencies.
- The primary valences are normally ionizable and are satisfied by negative ions.
- Secondary valences are non-ionizable. These are satisfied by negative ions. Also, the secondary valence is fixed for any metal and is equal to its coordination number.
- The ions bounded by the secondary linkages to the metal exhibit characteristic spatial arrangements corresponding to different coordination numbers.
He further postulated that octahedral, tetrahedral, and square planar geometrical shapes are more common in coordination compounds of transition metals. Thus, [Co(NH3)6]3+, [CoCl(NH3)5]2+ and [CoCl2(NH3)4]+are octahedral entities, while [Ni(CO)4] and [PtCl4]2– are tetrahedral andsquare planar, respectively.
Difference between Primary and Secondary Valency in Coordination Compounds:
Limitations of Werner’s Theory
While Werner's theory of coordination compounds was groundbreaking and highly influential, it also had certain limitations. Some of the key limitations of Werner's theory are as follows:
- Limited scope: Werner's theory primarily focused on coordination compounds involving transition metal ions and ligands. It did not adequately explain the bonding and structure of non-transition metal complexes or compounds involving non-ligand molecules.
- Neglect of electronic structure: Werner's theory did not take into account the electronic structure and configuration of metal ions and ligands. It focused more on the coordination number and geometric arrangements rather than considering the orbital interactions and electronic properties of the species involved.
- Inability to explain magnetic properties: Werner's theory was unable to fully explain the observed magnetic properties of coordination compounds. It did not account for the magnetic behavior arising from unpaired electrons in transition metal complexes.
- Lack of clarity on bonding: While Werner's theory introduced the concept of coordination bonds between ligands and metal ions, it did not provide a comprehensive understanding of the nature of these bonds. It did not address the extent of covalent and electrostatic interactions between the metal and ligands.
- Simplistic treatment of isomerism: Werner's theory recognized geometric and linkage isomerism but did not offer detailed explanations or predictions of these isomeric forms. It lacked a comprehensive framework for understanding the factors governing isomerism in coordination compounds.
Despite these limitations, Werner's theory laid the foundation for modern coordination chemistry and significantly advanced the understanding of coordination compounds.
Basic Concepts/Terms Related to Coordination Compounds:
Coordination compounds, also known as complex compounds, are substances that contain a central metal atom or ion bonded to one or more ligands. Here are some Basic Concepts/Terms Related to Coordination Compounds:
Coordination Entity
A chemical compound in which the central ion or atom (or the coordination center) is bound to a set number of atoms, molecules, or ions is called a coordination entity.
Examples: [CoCl3(NH3)3], and [Fe(CN)6]4-.
Central Atoms and Central Ions
The atoms and ions to which a set number of atoms, molecules, or ions are bound are referred to as the central atoms and the central ions. In coordination compounds, the central atoms or ions are typically Lewis Acids and can, therefore, act as electron-pair acceptors.
Ligands
The atoms, molecules, or ions that are bound to the coordination centre or the central atom/ion are referred to as ligands. These ligands can either be a simple ion or molecule (such as Cl– or NH3) or in the form of relatively large molecules, such as ethane-1,2-diamine (NH2-CH2-CH2-NH2).
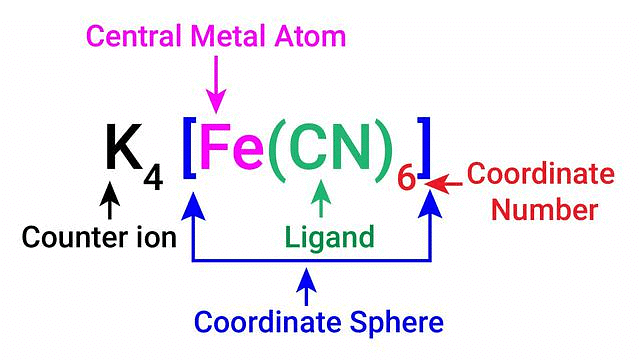
Coordination Number
The coordination number of the central atom in the coordination compound refers to the total number of sigma bonds through which the ligands are bound to the coordination centre.
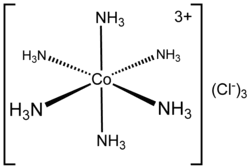
Example: [Co(NH3)6]3+, which features a 6-coordinate metal centre with octahedral molecular geometry, have coordination number 6
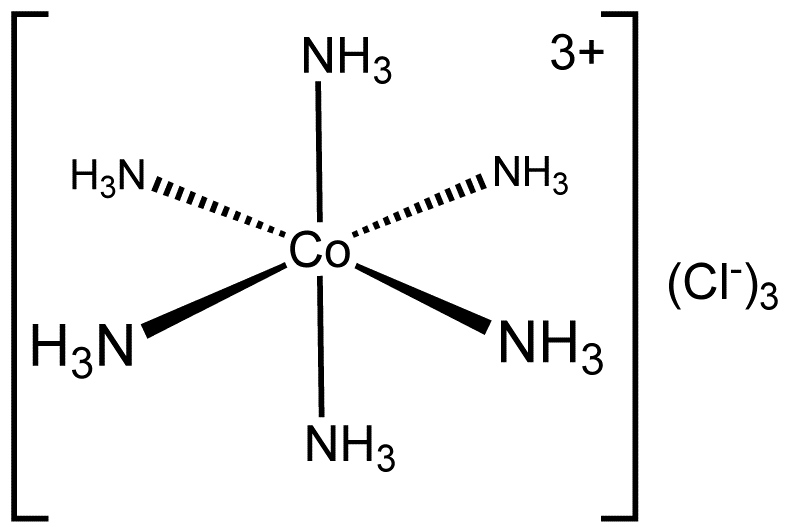
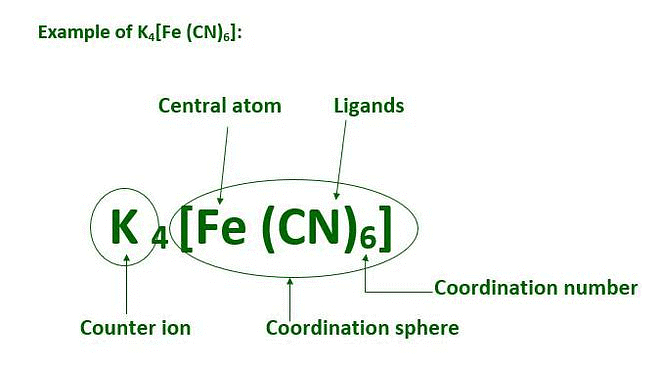
Coordination Sphere
The non-ionizable part of a complex compound consists of a central transition metal ion surrounded by neighbouring atoms or groups enclosed in a square bracket.
The coordination centre, the ligands attached to the coordination centre, and the net charge of the chemical compound as a whole, form the coordination sphere when written together. This coordination sphere is usually accompanied by a counter ion (the ionizable groups that attach to charged coordination complexes).
Example: In the complex K4[Fe(CN)6], [Fe(CN)6]4– is the coordination sphere and K+ is the counter ion.
Coordination Polyhedron
The geometric shape formed by the attachment of the ligands to the coordination centre is called the coordination polyhedron.
Example: The most common coordination polyhedra are octahedral, square planar and tetrahedral. For example, [Co(NH3)6]3+ is octahedral, [Ni(Co)4] is tetrahedral and [PtCl4]2– is square planar.
Oxidation Number
The oxidation number of the central atom can be calculated by finding the charge associated with it when all the electron pairs that are donated by the ligands are removed from it.
Example: The oxidation number of the platinum atom in the complex [PtCl6]2- is +4.
Homoleptic and Heteroleptic Complex
Homoleptic Complex: When the coordination centre is bound to only one type of electron pair donating ligand group, the coordination complex is called a homoleptic complex, for example: [Cu(CN)4]3-.
Heteroleptic Complex: When the central atom is bound to many different types of ligands, the coordination compound in question is called a heteroleptic complex, an example for which is [Co(NH3)4Cl2]+.
Double salts
Those which retain their identity in solutions are called double salts. For example.
Complex Coordination Compounds
Those who lose their identity in solution (complexes). For example.
When crystals of carnallite are dissolved in water, the solution shows properties of K+, Mg2+ and Cl- ions. In a similar way, a solution of potassium alum shows the properties of K+, Al3+ and SO42- ions. These are both examples of double salts which exist only in the crystalline state. When the other two examples of coordination compounds are dissolved they do not form simple ions, Cu2+, Fe2+ and CN-, but instead, their complex ions are formed.
Representation of Coordination Complexes
Coordination complexes are typically represented using various notations and symbols to describe their structures and compositions. They are represented by:
where,
- M = Central Metal atom /ion (usually of d-block)
- L = Ligand
- x = No. of ligands
= charge on coordination
Outside region apart from coordination sphere is called ionisation sphere.
1. Central metal atom/ion: Central ion acts as an acceptor (Lewis acid) and has to accommodate electron pairs donated by the donor atom of the ligand, it must have empty orbitals. This explains why the transition metals having empty d-orbitals form co-ordination compounds readily. Thus, in complexes [Ni(NH3)6]2 and [Fe(CN)6]3-, Ni2 and Fe3 respectively are the central metal ions.
2. Ligands: Species that are directly linked with the central metal atom/ ion in a complex ion are called ligands. The ligands are attached to the central metal atom /ion through co-ordinate or dative bond free ligands that have at least one lone pair.
The ligands are thus Lewis bases and the central metal ions and n atoms are Lewis acids.
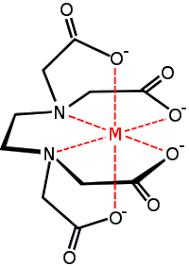 Ligands
Ligands
Ligands can be of the following types depending on the number of donor atoms pesent in them.
(i) Mono / Unidentate Ligands They have one donor atom, i.e., they can donate only one electron pair to the central metal atom /ion eg., F-, Cl-, Br-, H2O, NH3, CN-,NO2-, OH-,
CO etc.
 Unidentate Ligands
Unidentate Ligands
(ii) Bidentate Ligands Ligands which have two donor atoms and have the ability to link with the central metal atom /ion at two position are called bidentate ligands e.g.
(iii) Tridentate Ligands Ligands having three donor atoms are called tridentate ligands. Examples are
diethylenetriamine (dien)
(iv) Tetradentate Ligands These ligands possess four donor atoms. Examples are
(v) Pentadentate Ligands They have five donor atoms. For example, ethylenediamine triacetate ion.
(vi) Hexadentate Ligands They have six donor atoms. The most important example is the ethylenediaminetetraacetate ion.
(vii) Ambidentate ligands: There are certain ligands that have two or more donor atoms but in forming complexes, only one donor atom is attached to the metal/ion. Such ligands are called ambidentate ligands. Some examples of such ligands are
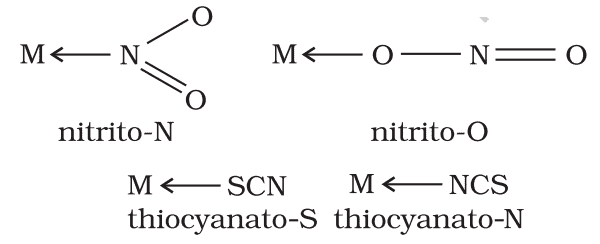
(viii) Ligands having more than two donor atoms are called polydentate or multidentate ligands. Multidentate ligands are known as a chelating ligand, it results in the formation of a stable cyclic ring thus, the complexes formed are also called chelates. Chelating ligands are usually organic compounds.
3. Co-ordination sphere The central metal atom and the ligands directly attached to it are collectively termed as the coordination sphere. The coordination sphere is written inside square brackets, for examples, [Co(NH3)6]3+. Remember that the central metal atom and the ligands inside the square brackets, behave as a single entity.
4. Co-ordination number (CN) The coordination number (CN) of a metal atom /ion in a complex is the total number of e- pairs accepted by central metal atom /ion from ligands through the coordinate bond.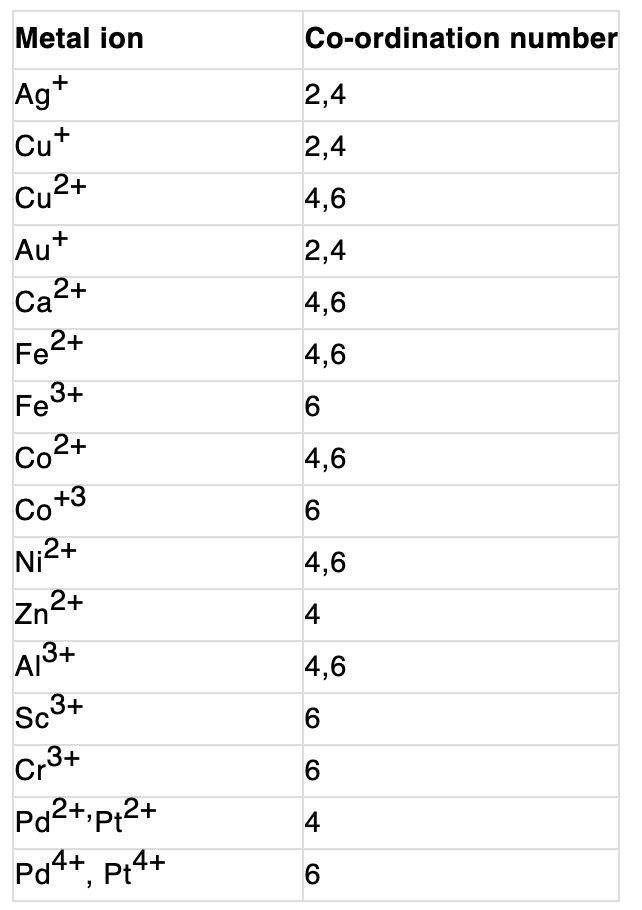

5. Oxidation number/oxidation state (O.S.) of central metal ion It is a number(numerical value) that represents the electric charge on the central metal atom of a complexion. for example the oxidation number of Fe, CO and Ni in [Fe(CN)6]4-, [Co(NH3)6]3 and Ni(CO)4 are 2, 3 and zero, respectively. Let us take a few examples to illustrate this.
(i) Potassium Ferrocyanide, K4[Fe(CN)6] Since the complex has four monovalent cations outside the coordination sphere, the complex ion must carry four negative charges, i.e., it is [Fe(CN)6]4-. The number of CN- ion (univalent ion), that is 6 represents the coordination number of Fe cation. The oxidation state of iron can be determined easily as below, knowing that cyanides ions are unidentate and the complex, on the whole, carries a -4 charge.
[Fe(CN)6]-4
x + (-6) = -4
Therefore, x = 2
Thus, here iron is present as Fe2+ or Fe(II).
(ii) [Cr(C2O4)3]3- Note that here the oxalate ligand is a negative ion, that is it is bidentate. Therefore three oxalate ligands carry a total charge of -6 and the coordination number of Cr is 6. Now since the complex carries a -3 charge, therefore the oxidation state of Cr is 3.
(iii) Ni(CO)4 Here the coordination number of Ni is 4 since the carbonyl group is unidentate. Further, since the complex, as well as the ligands, has no charge, the nickel atom must also be neutral, that is it is in zero oxidation state.
6. Effective atomic number - EAN (Sidgwick Theory and EAN Rule): Total no. of electrons present on central metal atom /ion. after accepting electron pairs from donor atom of ligands through coordinate bond is called E.A.N. of central metal atom /ion.
Sidgwick also suggested that the metal ion will continue accepting electron pairs till the total number of electrons in the metal ion and those donated by ligands is equal to that
of a nearest noble gas. This total number of electrons is called the effective atomic number (EAN) of the metal /ion.
This will become clear by taking the example of hexamine cobalt (III) ion [Co(NH3)6]3
The atomic number of cobalt = 27
In the present complex, cobalt is present in the oxidation state of 3.
Therefore, E.A.N. of Co3+ = Z - O.S. + 2 × C.N.
= 27 - 3 + 2 × 6 = 36
In the above example since the number 36 corresponds to the atomic number of krypton, according to Sidgwick, the complex will be stable. Though the EAN rule (which states that those complexes are stable whose EAN is the same as the atomic number of the next noble gas) is applicable in many metal carbonyl complexes, however, there are several examples in which the EAN rule is not obeyed.
|
75 videos|278 docs|78 tests
|
FAQs on Werner’s Theory & Some Basic Concepts of Coordination Compounds - Chemistry Class 12 - NEET
| 1. What is Werner’s Theory of Coordination Compounds? |  |
| 2. What were the key experiments conducted by Werner to support his theory? |  |
| 3. What are the main postulates of Werner’s Theory? |  |
| 4. What are the limitations of Werner’s Theory? |  |
| 5. How are coordination complexes represented in chemical notation? |  |


















