Lakhmir Singh & Manjit Kaur Solutions: Acids, Bases & Salts - 2 | Science Class 10 PDF Download
(Page No - 68)
Question 31:
(a) What happens when an acid reacts with a metal carbonate ? Explain with the help of an example. Write
chemical equation of the reaction involved.
(b) What happens when carbon dioxide gas is passed through lime water :
(i) for a short time ?
(ii) for a considerable time ?
Write equations of the reactions involved.
Solution :
(a) When an acid reacts with a metal carbonate, then a salt, carbon dioxide and water are produced.
Example: When dilute hydrochloric acid reacts with sodium carbonate, then sodium chloride, carbon dioxide and water are formed.
Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + C02(g) + H20(l)
(b) (i) Lime water turns milky.
CaOH)2 (aq) + CO2 (g) → CaCO3(s) + H20(l)
Calciumhydroxide carbon dioxide Calciumcarbonate Water
(Lime water) (White ppt.)
(Makes limewater milky)
(ii) Lime water solution becomes clear.
CaC03(s) + C02(g) + H20(l)→ Ca(HC03)2(aq)
Question 32:
With the help of labelled diagrams, describe an activity to show that acids produce ions only in aqueous solutions.
Solution :
Activity:
Take about 1g solid NaCl in a clean and dry test tube and add some concentrated sulphuric acid to it. Fit a rubber cork with a small delivery tube in the mouth of the test tube. Concentrated sulphuric acid reacts with sodium chloride to form hydrogen chloride gas. The hydrogen chloride gas starts coming out of the open end of the glass tube.
Now, hold a ‘dry’ blue litmus paper in HCl gas. There is no change in colour of the ‘dry’ blue litmus paper. This shows that HCl gas does not behave as an acid in the absence of water. However, when we hold a ‘moist’ blue litmus paper in HCl gas, we will see that the ‘moist’ blue litmus paper turns red. This indicates that HCl gas shows acidic behavior in the presence of water as hydrogen ions are formed. This proves that acids produce ions only in aqueous solutions or in presence of water.
Question 33:
(a) Which element is common to all acids ?
(b) Compounds such as alcohol and glucose also contain hydrogen but are not categorised as acids. Describe an activity to prove it.
Solution :
(a) Hydrogen.
(b) Activity:
Take solutions of glucose, alcohol, hydrochloric acid and sulphuric acid. Fix two nails on a cork, and place the cork in a 100 ml beaker. Connect the nails to the two terminals of a 6 volt battery through a bulb and a switch. Now pour some dilute HCl in the beaker and switch on the current. The bulb starts glowing. This shows that HCl solution taken in the beaker conducts electricity. If we replace hydrochloric acid with sulphuric acid and perform the experiment, the bulb would glow again. This shows that an aqueous solution of an acid conducts electricity due to the presence of charged particles called ions in it.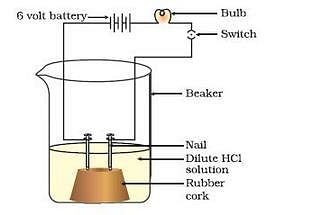
Now, if we take glucose solution in the beaker and switch on the current, the bulb would not glow. If we repeat the experiment by taking alcohol solution in the beaker, the bulb would not glow again. This shows that due to the absence of ions, glucose and alcohol solutions do not conduct electricity. From this activity, we conclude that the hydrogen containing compounds such as glucose and alcohol are not categorised as acids because they do not dissociate (or ionise) in water to produce hydrogen ions [H+(aq) ions].
Question 44:
When a piece of limestone reacts with dilute HCl, a gas X is produced. When gas X is passed through lime water then a white precipitate Y is formed. On passing excess of gas X, the white precipitate dissolves forming a soluble compound Z.
(a) What are X, Y and Z ?
(b) Write equations for the reactions which take place :
- when limestone reacts with dilute HCl
- when gas X reacts with lime water to form white precipitate Y
- when excess of gas X dissolves white precipitate Y to form a soluble compound Z
Solution :
(a) X is carbon dioxide; Y is calcium carbonate; Z is calcium hydrogen carbonate.
(b) (i) CaC03+ → CaCl2 H20+C02
(ii) Ca(OH)2(aq) +C02 (g) → CaC03(s) H20(l)
(iii) CaC03 (s) + C02 (g) + H20(l) → Ca(HC03)2 (aq)
Question 45:
If someone is suffering from the problem of acidity after overeating, which of the following would you suggest as remedy ?
Lemon juice, Vinegar, Baking soda solution Give reason for your choice.
Solution :
Baking soda solution. Being basic in nature, it neutralises excess acid in the stomach.
(Page No - 69)
Question 46:
On adding dilute hydrochloric acid to copper oxide powder, the solution formed is blue-green.
(a) Predict the new compound formed which imparts a blue-green colour to solution.
(b) Write a balanced chemical equation of the reaction which takes place.
(c) On the basis of the above reaction, what can you say about the nature of copper oxide ?
Solution :
(a) Copper (II)
chloride, CuCl2
(b) CuO(s0+ 2HCl(aq) --> CuCl2(aq) + H2O(I)
(c) Copper oxide is basic in nature
Question 47:
A white shirt has a yellow stain of curry. When soap is rubbed on this shirt during washing, the yellow stain turns reddish-brown. On rinsing the shirt with plenty of water, the reddish-brown stain turns yellow again.
(a) Name the natural indicator present in curry stain.
(b) Explain the changes in colour of this indicator which take place during washing and rinsing the shirt.
(c) What is the nature of soap (acidic/basic) as shown by the indicator present in curry stain ?
Solution :
(a) Turmeric.
(b) The yellow stain of curry turns reddish-brown when soap is scrubbed on it because of the fact that soap solution is basic in nature which changes the colour of turmeric in the curry stain to red-brown. This stain turns yellow again when the cloth is rinsed with water because then the basic soap gets removed with water.
(c) Basic.
Question 48:
You have been provided with three test-tubes. One of these test-tubes contains distilled water and the other two contain an acidic and a basic solution respectively. If you are given only blue litmus paper, how will you identify the contents of each test-tube ?
Solution :
Acidic
solution will turn blue litmus red; This red litmus will turn blue in basic solution; Distilled water will have no effect on any type of litmus paper.
Question 49:
A substance X which is used as an antacid reacts with dilute hydrochloric acid to produce a gas Y which is used in one type of fire-extinguisher. Name the substance X and gas Y. Write a balanced equation for the chemical reaction which takes place.
Solution :
Substance X is sodium hydrogen carbonate; Gas Y is carbon dioxide.
NaHCO3(s) + HCl(aq) --> CO2(g) + NaCl(aq) + H2O(l)
Question 50:
How is the neutralisation of a carbonate with an acid different from the neutralisation of an oxide or a hydroxide ?
Solution :
Neutralisation of a carbonate with an acid produces carbon dioxide gas but not with an oxide or hydroxide.
Question 51:
What happens to (a) the H+ ions, and (b) temperature of the solution, when an acid is neutralised ?
Solution :
(a) H+ ions of acid combine with OH– ions of alkali to form water, H2O.
(b) Temperature of the solution rises.
(Page No - 79)
Question 1:
Name the gas evolved when zinc granules are treated/heated with :
(a) hydrochloric acid solution
(b) sodium hydroxide solution
Solution :
(a) Hydrogen
(b) Hydrogen
Question 2:
What is the common name of water soluble bases ?
Solution :
Alkalis
Question 3:
What is common in all the water soluble bases (or alkalis) ?
Solution :
They all produce hydroxide ions when dissolved in water.
Question 4:
Why does tooth decay start when the pH of mouth is lower than 5.5 ?
Solution :
Tooth decay start when the pH of mouth is lower than 5.5 because the acid becomes strong enough to attack the enamel of the teeth and corrode it.
Question 5:
What is the pH of a neutral solution ?
Solution :
7
Question 6:
Which is more acidic : a solution of pH = 2 or a solution of pH = 6 ?
Solution :
Solution of pH = 2 is more acidic.
Question 7:
Which is more basic (or more alkaline) : a solution of pH = 8 or a solution of pH = 11 ?
Solution :
Solution of pH = 11
Question 8:
Name the scientist who developed the pH scale.
Solution :
Sorenson
Question 9:
Name the indicator which can give us an idea of how strong or weak an acid or base is.
Solution :
Universal indicator
Question 10:
The pH of soil A is 7.5 while that of soil B is 4.5. Which of the two soils, A or B, should be treated with powdered chalk to adjust its pH and why ?
Solution :
Soil B. Soil B is acidic in nature so its treated with powdered chalk to reduce its acidity.
Question 11:
What is the name of the indicator which can be used for testing the pH of a solution ?
Solution :
Universal indicator
Question 12:
What colour will universal indicator show if you add it to the following substances ?
(a) potassium hydroxide, pH = 12
(b) soda water, pH = 5
(c) sulphuric acid, pH = 2
Solution :
(a) Dark Purple
(b) Orange Yellow
(c) Red
Question 13:
A beaker of concentrated hydrochloric acid has a pH of 1. What colour will full range universal indicator turn if it is added to this beaker ? Is it a strong or a weak acid ?
Solution :
pH = 1 will turn the scale red; strong acid.
Question 14:
Two solutions X and Y are tested with universal indicator. Solution X turns orange whereas solution Y turns red. Which of the solutions is a stronger acid ?
Solution :
Solution Y is a stronger acid.
Question 15:
Two solutions A and B have pH values of 3.0 and 9.5 respectively. Which of these will turn litmus solution from blue to red and which will turn phenolphthalein from colourless to pink ?
Solution :
Solution A (pH = 3.0) will turn litmus from solution blue to red Solution B (pH = 9.5) will turn phenolphthalein from colourless to pink.
Question 16:
Two drinks P and Q gave acidic and alkaline reactions, respectively. One has a pH value of 9 and the other has a pH value of 3. Which drink has the pH value of 9 ?
Solution :
Drink Q has a pH value of 9.
Question 17:
Two solutions X and Y have pH = 4 and pH = 8, respectively. Which solution will give alkaline reaction and which one acidic ?
Solution :
Alkaline reaction: Solution Y (pH = 8)
Acidic reaction: Solution X (pH = 4)
Question 18:
Fill in the following blanks with suitable words :
(a) Acids have a pH………… than 7.
(b) Alkalis have a pH………. than 7.
(c) Neutral substances have a pH of……………
(d) The more acidic a solution, the………………. the pH.
(e) The more alkaline a solution, the…………… the pH.
Solution :
(a) Lower.
(b) Higher.
(c) 7.
(d) Lower.
(e) Higher.
Question 19:
Fresh milk has a pH of 6. When it changes into curd (yogurt), will its pH value increase or decrease ? Why ?
Solution :
pH value will decrease when milk changes to curd. Curd contains lactic acid hence the pH decreases.
Question 20:
(a) What is a universal indicator ? For what purpose is it used ?
(b) How does a universal indicator work ?
(c) Water is a neutral substance. What colour will you get when you add a few drops of universal indicator to a test-tube containing water ?
Solution :
(a) Universal indicator is a mixture of many different indicators which gives different colours at different pH values of the entire pH scale. It is used to obtain an idea of how acidic or basic a substance is.
(b) When an acid or base solution is added to the universal indicator, it produces a new colour which is used to find the pH value of the acid or the base solution by matching the colour with the colours on pH colour chart.
(c) Green colour.
Question 21:
Which chemical is injected into the skin of a person :
(a) during an ant’s sting ?
(b) during the nettle leaf hair sting ?
How can the effect of these stings be neutralised ?
Solution :
(a) Methanoic acid.
(b) Methanoic acid.The effect of methanoic acid can be neutralised by rubbing a mild base like baking soda solution on the stung area of the skin.
|
85 videos|437 docs|75 tests
|
FAQs on Lakhmir Singh & Manjit Kaur Solutions: Acids, Bases & Salts - 2 - Science Class 10
| 1. What are acids and bases? |  |
| 2. What is the pH scale and how is it related to acids and bases? |  |
| 3. How can we differentiate between acids and bases using indicators? |  |
| 4. What are the common properties of acids? |  |
| 5. How do acids and bases react with each other to form salts? |  |

|
Explore Courses for Class 10 exam
|

|


















