Solubility: Solid, Gas & Liquid in a Liquid and Henry's Law | Chemistry Class 12 - NEET PDF Download
What is Solubility?
The maximum amount of solute that can dissolve in a known quantity of solvent at a certain temperature is its solubility.
A solution is a homogeneous mixture of one or more solutes in a solvent. Sugar cubes added to a cup of tea or coffee is a common example of a solution. The property which helps sugar molecules to dissolve is known as solubility. Hence, the term solubility can be defined as a property of a substance (solute) to dissolve in a given solvent. A solute is any substance that can be either solid or liquid or gas dissolved in a solvent.
 On this basis, the factors affecting solubility vary on the state of the solute:
On this basis, the factors affecting solubility vary on the state of the solute:
- Solids in Liquids
- Liquids in Liquids
- Gases in Liquids
The maximum amount of solute which can be dissolved in a specified amount of solvent at a given temperature is called the solubility of a substance (solute).
Dissolution
Dissolution means to dissolve. When a solid is added to a solvent (liquid); the concentration of solution increases because of dissolving of some of the solute. This process is called dissolution.

Crystallisation
In the process of dissolution, some of the particles of solute collide with the solid particles of solute and separated out of the solution, which is called crystallization.

A stage is reached when the two processes occur at the same rate. Under such conditions, a number of solute particles going into the solution will be equal to the solute particles separating out and a state of dynamic equilibrium is reached.
Dynamic Equilibrium
In the process of dissolution and crystallization, the stage in which the rate of both of the process started and keeps on occurring at the same rate is called the dynamic equilibrium.
In the condition of dynamic equilibrium number of solid particles mixing in the solution becomes equal to the solute particles separating out from the solution.
The state of dynamic equilibrium can be shown by using the following equation:
| Solute + Solvent ⇆ Solution |
A similar process occurs when a gas is dissolved in liquid.
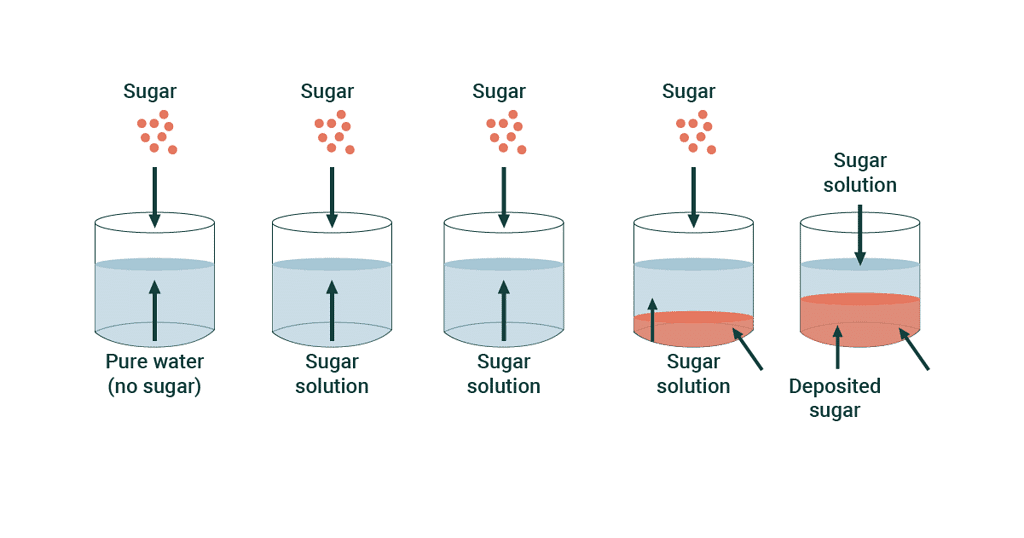
Saturated Solution
At the stage of dynamic equilibrium the concentration of solute remains constant in solution under given temperature and pressure, is called saturated solution.
The solution in which no more solute can be dissolved under the given conditions, i.e. temperature and pressure are called a saturated solution.
Unsaturated Solution
The solution in which more solute can be dissolved under the given conditions, i.e. temperature and pressure, is called unsaturated solution.

1. Solubility of a Solid in a Liquid
Conditions for a solute to dissolve in a solvent
- Solute and solvent both should be polar or non-polar.
- The intermolecular attractions of both the solute and solvent should be similar.
Factors Affecting Solubility of a Solid in a Liquid
Nature of Solute and Solvent
Like Dissolves Like Every solid does not dissolve in every liquid. Only polar solute (solid) dissolves in polar liquid (solvent) and non-polar solute (solid) dissolved in non-polar liquid(solvent).
If intermolecular attractions of solute and solvent are similar, then that solute (solid) dissolve in that very solvent.
Example: Sugar and common salt readily dissolve in water while naphthalene and anthracene do not. Naphthalene and anthracene dissolve in benzene while common salt and sugar do not.Effect of Temperature:
Apart from the nature of solute and solvent, temperature also affects solid solubility considerably.
If the dissolution process is endothermic (∆H > 0), then the solubility should increase with an increase in temperature in accordance with Le Chatelier’s Principle.
If the dissolution process is exothermic (∆H < 0) the solid solubility should decrease.Effect of Pressure:
Solid solubility hardly gets affected by changes in pressure. This is due to the fact that solids and liquids are highly incompressible and practically do not get affected by changes in pressure.
2. Solubility of Gases in Liquids
Gas solubility in liquids deals with the concept of gas dissolving in a solvent. Let us first define solubility.

For any substance, solubility is the maximum amount of solute that can be dissolved in a given solvent at a particular temperature.
- Now our concern is gas solubility in liquids. The gas solubility in liquids is greatly affected by temperature and pressure as well as the nature of the solute and the solvent.
- The common examples are carbonated beverages, i.e., soft drinks, household cleaners containing aqueous solutions of ammonia, a formalin-an aqueous solution of formaldehyde, etc.
- The natural waters contain dissolved; O2 which is vital for the existence of aquatic life in the sea, rivers and lakes.
- There are many gases that readily dissolve in water, while there are gases that do not dissolve in water under normal conditions. Oxygen is only sparingly soluble in water while HCl or ammonia readily dissolves in water.
The solubility of a gas in a liquid is expressed in terms of the absorption coefficient. It is defined as the volume of the gas in mL that can be dissolved by 1 mL of a liquid solvent at the temperature of the experiment at one atmospheric pressure.
The volume of the gas is measured at STP. Thus, if v is the volume of the gas dissolved, reduced to STP, V is the volume of the solvent and P is the pressure of the gas in atmospheres, then the absorption coefficient, a, is given by α = v/VP
Factors Affecting Solubility of a Gases in a Liquid
Effect of Pressure:
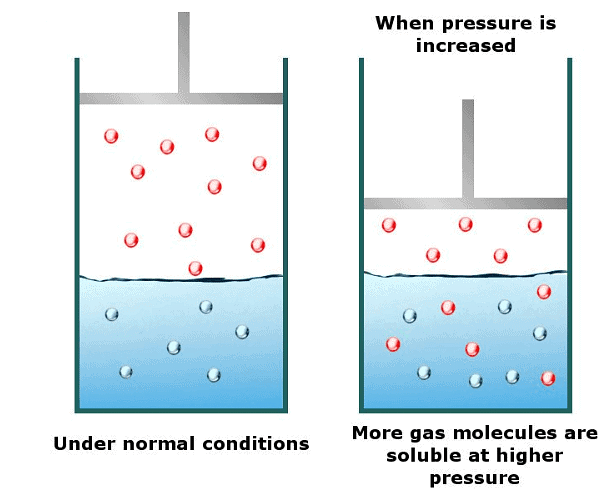
It has been found that the gas solubility in liquids increases with an increase in pressure. To have a better understanding of the effect of pressure on gas solubility let us consider a system of a gas solution in a solvent in a closed container in a state of dynamic equilibrium.- Now the solution is in equilibrium and hence the rate of gaseous molecules entering the solution is equal to the rate of gaseous molecules leaving the solution.
- Now suppose we increase the pressure of the system by compressing the gas molecules present in the solution. As a result of an increase in pressure, the gases molecules will now be concentrated in a smaller volume. This will result in an increase in the number of gas molecules per unit volume available above the solution.
- Since the number of gas molecules presents above the solution has increased, the rate with which the gas molecules will be entering the solution will also increase.
- The end result is an increase in the number of gas molecules in the solution until a new equilibrium point is attained. Thus the solubility of gases increases with an increase in the pressure of a gas above the solution.
Effect of Temperature:
Gas solubility in liquids is found to decrease with an increase in temperature. The gas molecules in a liquid are dissolved by the process of dissolution.- During this process, heat is evolved. According to Le Chatelier’s Principle which states that when the equilibrium of a system is disturbed, the system readjusts itself in such a way that the effect that has caused the change in equilibrium is countered.
- So, as we know that dissolution is an exothermic process, the solubility should decrease with an increase in temperature to validate Le Chatelier’s Principle.
Nature of the gas and solvent:
Generally, the gases which can be easily liquefied are more soluble in common solvents.- Example: CO2 is more soluble in water than oxygen or hydrogen. The gases which react with the solvent possess higher solubility.
- HCI and NH3 are highly soluble in water. Oxygen, nitrogen and carbon dioxide are much more soluble in ethyl alcohol than in water at the same temperature and pressure.
3. Solubility of Liquids In Liquids
Water is often called the "universal solvent" because it can dissolve many different substances, except for a few exceptions. The amount of a substance that can dissolve in a certain amount of water at a specific temperature is called its solubility. Solubility is like a new friendship formed between the molecules of the substance being dissolved and the water molecules. We measure solubility in grams per liter (g/L).
We classify substances based on how much of them can dissolve in water. If 0.1 grams or more of a substance can dissolve in 100 milliliters of water, we call it soluble. If it's less than 0.1 grams, we call it sparingly soluble. So, solubility tells us how much of a substance can dissolve in water.
Depending on solubility, we can have different types of solutions. A saturated solution is one where you've added as much of a substance as possible to the water, and it's all dissolved. But sometimes, you can dissolve even more than expected, creating a supersaturated solution. In a supersaturated solution, the extra substance might start forming solids or crystals because there's so much of it dissolved.
Factors Affecting Solubility of Liquid in Liquids
The solubility of a substance depends on the physical and chemical properties of that substance. In addition to this, there are a few conditions that can manipulate it. Temperature, pressure and the type of bond and forces between the particles are few among them.

Temperature:
In most cases, the solubility of liquids in liquids tends to increase with an increase in temperature, as higher temperatures usually provide more kinetic energy for the solvent molecules to overcome intermolecular forces and dissolve the solute.Forces and Bonds:
Chemical interactions such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces between the solute and solvent molecules can influence solubility. For instance, alcohol molecules (solutes) can form hydrogen bonds with water molecules (solvent), enhancing their solubility in water.Pressure:
Unlike gas solubility, pressure typically has a negligible effect on the solubility of liquids in liquids, except for systems where the solute or solvent is compressible.Nature of Liquids:
The chemical nature and polarity of both the solute and solvent play a significant role. Generally, like dissolves like, meaning polar solvents tend to dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes more readily.
Henry's Law
Henry’s Law gives a quantitative relation between pressure and gas solubility in a liquid. It states that:
The solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of liquid or solution.
The solubility is taken as the mass of the gas dissolved per unit volume of the liquid. Thus, if m is the mass of the gas dissolved per unit volume of the solvent and P is the pressure of the gas in equilibrium with the solution, then
| m ∝ p m = Kp |
where K is the proportionality constant.
When P = 1, m = K, i. e., the solubility of the gas at unit pressure is equal to constant K.

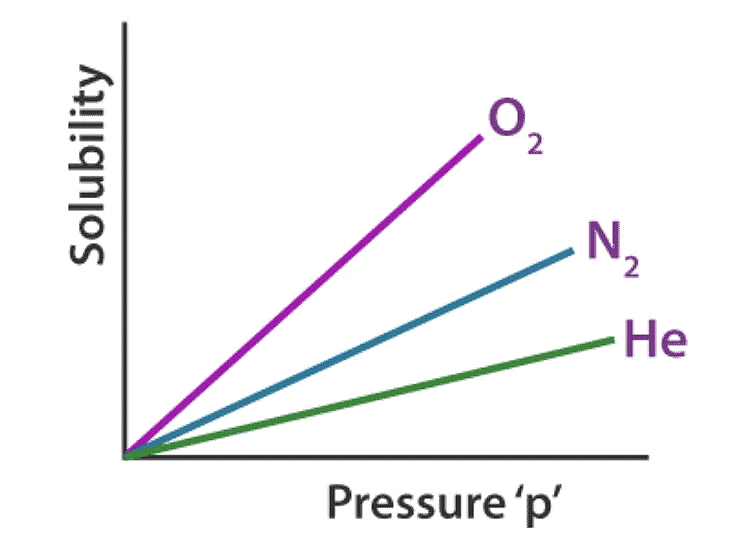
Factors Affecting the Henry’s Law Constant
- Nature of the gas: Different gases have varying solubilities in a solvent due to differences in molecular size, polarity, and intermolecular forces. For example, highly polar gases tend to be more soluble in polar solvents compared to nonpolar gases.

- Nature of the solvent: The solvent's polarity and molecular structure influence its ability to dissolve gases. Polar solvents, such as water, favor the dissolution of polar gases, while nonpolar solvents prefer nonpolar gases. Additionally, solvent-solute interactions affect the solubility.
- Temperature: Henry's Law constant is temperature-dependent. Generally, solubility decreases with increasing temperature due to decreased gas solvation in the solvent. However, this relationship can vary depending on the specific solute-solvent system and the enthalpy of solution.
- The units of pressure
Therefore, different gases have different Henry’s laws constant in different solvents, as illustrated graphically above.
Effect of Pressure on Solubility of Gases in Liquids
- Solubility of a gas in liquid increases with an increase in pressure and vice versa. That is the solubility of a gas in liquid decreases with a decrease in pressure.

- The solubility of a gas in liquid decreases with an increase in Henry’s Law constant (KH), at a given pressure. This means at a given pressure higher the value of KH (Henry’s Law constant), the lower is the solubility of the gas in a liquid and vice versa.
Example 1. If N2 gas is bubbled through water at 293 K, how many millimoles of N2 gas would dissolve in 1 litre of water? Assume that N2 exerts a partial pressure of 0.987 bar. Given that Henry’s law constant for N2 at 293 K is 76.48 kbar.
Solution.
The solubility of gas is related to the mole fraction in aqueous solution.
The mole fraction of the gas in the solution is calculated by applying Henry’s law.Thus:
x (Nitrogen) = p(nitrogen)/KH = 0.987 bar/76.480 = 1.29 × 10-5
As 1 litre of water contains 55.5 mol of it, therefore if n represents number of moles of N2 in solution,
x (Nitrogen) = n mol/n mol + 55.5 mol = n/55.5 = 1.29 × 10-5
(n in denominator is neglected as it is < < 55.5)
Thus n = 1.29 × 10–5 × 55.5 mol = 7.16 × 10–4 mol
Applications of Henry's Law
- Pepsi and other Carbonated Drinks: Since the solubility of a gas in liquid increases with an increase in pressure, hence increasing the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
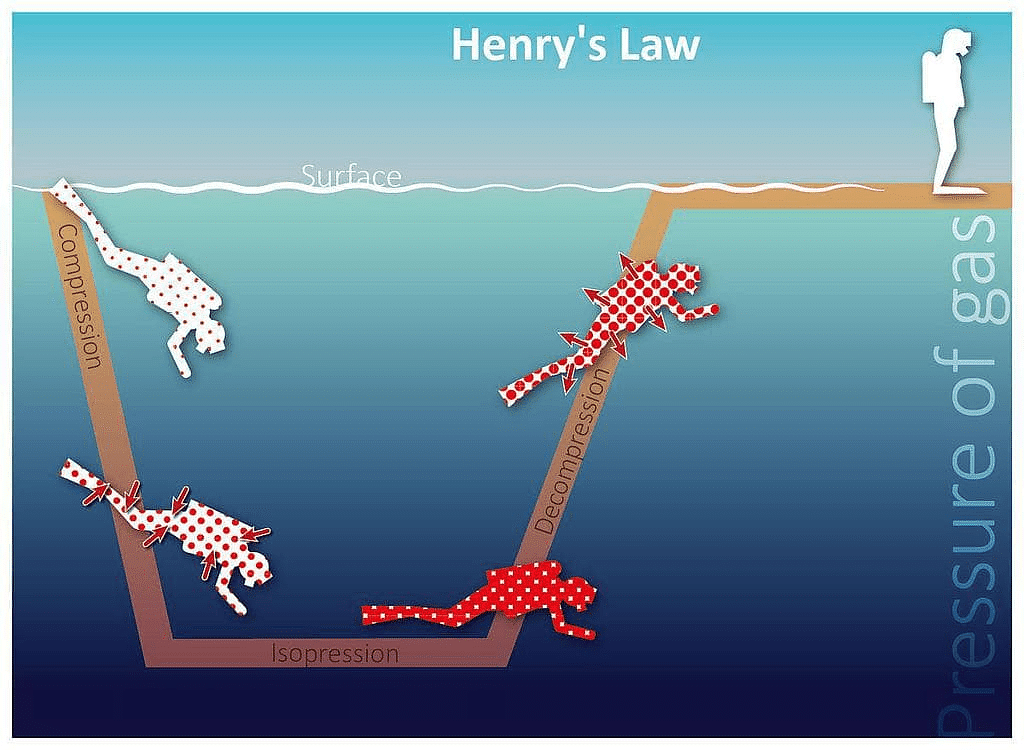
- Scuba divers have to face the problems of bends. When they dive and go deeper in water, pressure gradually increases resulting in an increase in the solubility of atmospheric gases in the blood. Bends is a medical condition which blocks the blood capillaries due to the formation of bubbles of nitrogen in the blood. Bends is very painful and dangerous to live.
When scuba divers come towards the surface of the water they have to face bends. Hence scuba diver uses a tank filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen) to avoid bends as well as toxic effects of high concentration of nitrogen in the blood because of increase in pressure underwater and decreasing pressure towards the water surface.
- People, who live at high altitudes and climbers (mountaineers) have to face a problem called anoxia. Anoxia is also a medical condition.
 The partial pressure of oxygen in the atmosphere decreases with high altitude. A decrease in partial pressure of oxygen leads to a low concentration of oxygen in blood and tissues. Because of low concentration of oxygen people feel short of breath and become weak and unable to think clearly.To avoid the condition of anoxia, mountaineers use oxygen cylinder while climbing to high altitude.
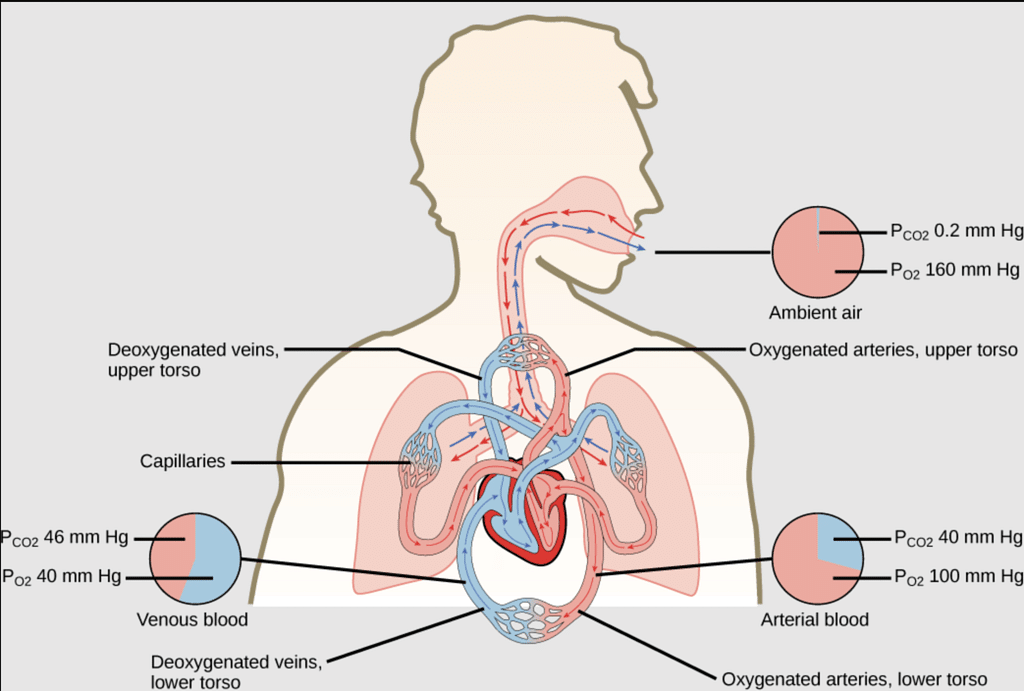
The partial pressure of oxygen in the atmosphere decreases with high altitude. A decrease in partial pressure of oxygen leads to a low concentration of oxygen in blood and tissues. Because of low concentration of oxygen people feel short of breath and become weak and unable to think clearly.To avoid the condition of anoxia, mountaineers use oxygen cylinder while climbing to high altitude. - Respiration and the Oxygenation of Blood: In the lungs, where oxygen is present in the air with high partial pressure, haemoglobin combines with oxygen to form oxyhaemoglobin. In tissues where the partial pressure of oxygen is low, oxyhaemoglobin releases oxygen for utilization in cellular activities.
 Respiration and the Oxygenation of Blood
Respiration and the Oxygenation of Blood
Limitations of Henry's Law
Henry's law holds good if the following conditions are fulfilled:
- The pressure is not too high,
- The temperature is not very low,
- The gas does not chemically combine with the solvent.
Example.2. Calculate the concentration of CO2 in a soft drink that is bottled at partial pressure of CO2 of 4 atm' over the liquid at 25° C. The Henry’ Law constant for CO2 in water at 25 ˚C is 3.1 × 10-2 mol/litre-atm.
Solution.
According to Henry's Law:
S = KP
3.1 × 10-2 × 4 = 0.12 mol litre-1
Try Yourself!
Q.1. At 20° C the solubility of nitrogen gas in water is 0.0150 g/litre when the partial pressure N2 is 580 torr. Find the solubility N2 in H2O at 20°C when its partial pressure is 800 torr.
Ans. 0.0207 g/litre.
|
100 videos|282 docs|123 tests
|
FAQs on Solubility: Solid, Gas & Liquid in a Liquid and Henry's Law - Chemistry Class 12 - NEET
| 1. What is solubility? |  |
| 2. How does solubility of a solid in a liquid vary with temperature? |  |
| 3. What factors can affect the solubility of gases in liquids? |  |
| 4. How does the solubility of liquids in liquids vary? |  |
| 5. What are some factors that can affect the solubility of a substance in a liquid? |  |

|
Explore Courses for NEET exam
|

|
 The partial pressure of oxygen in the atmosphere decreases with high altitude. A decrease in partial pressure of oxygen leads to a low concentration of oxygen in blood and tissues. Because of low concentration of oxygen people feel short of breath and become weak and unable to think clearly.To avoid the condition of anoxia, mountaineers use oxygen cylinder while climbing to high altitude.
The partial pressure of oxygen in the atmosphere decreases with high altitude. A decrease in partial pressure of oxygen leads to a low concentration of oxygen in blood and tissues. Because of low concentration of oxygen people feel short of breath and become weak and unable to think clearly.To avoid the condition of anoxia, mountaineers use oxygen cylinder while climbing to high altitude.