Solubility Equilibria of Sparingly Soluble Salts | Chemistry Class 11 - NEET PDF Download
Solubility Product (Ksp)
The solubility product constant is the equilibrium constant for the dissolution of a solid substance into an aqueous solution. It is denoted by the symbol Ksp.
The solubility product is a kind of equilibrium constant and its value depends on temperature. Ksp usually increases with an increase in temperature due to increased solubility.
Solubility is defined as a property of a substance called solute to get dissolved in a solvent in order to form a solution. The solubility of ionic compounds (which disassociate to form cations and anions) in water varies to a great deal. Some compounds are highly soluble and may even absorb moisture from the atmosphere whereas others are highly insoluble.
Significance of Solubility Product
Solubility depends on a number of parameters amongst which lattice enthalpy of salt and solvation enthalpy of ions in the solution are of most importance.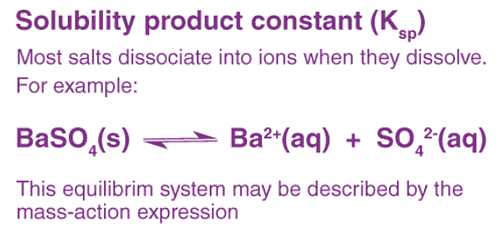
- When a salt is dissolved in a solvent the strong forces of attraction of solute (lattice enthalpy of its ions) must be overcome by the interactions between ions and the solvent.
- The solvation enthalpy of ions is always negative which means that energy is released during this process.
- The nature of the solvent determines the amount of energy released during solvation that is solvation enthalpy.
- Non-polar solvents have a small value of solvation enthalpy, meaning that this energy is not sufficient to overcome the lattice enthalpy.
- So the salts are not dissolved in non-polar solvents. Hence, for salt to be dissolved in a solvent, its solvation enthalpy should be greater than its lattice enthalpy.
- Solubility depends on temperature and it is different for every salt.
Salts are classified on the basis of their solubility in the following table: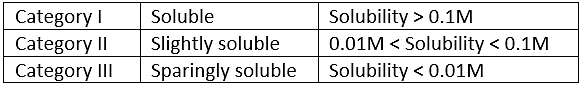
Solubility Product Constant
Suppose barium sulphate along with its saturated aqueous solution is taken.
The following equation represents the equilibrium set up between the undissolved solids and ions:
The equilibrium constant in the above case is: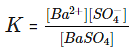
In case of pure solid substances the concentration remains constant, and so we can say:
Here Ksp is known as the solubility product constant. This further tells us that solid barium sulphate when in equilibrium with its saturated solution, the product of concentrations of ions of both barium and sulphate is equal to the solubility product constant.
Factors affect the value of Ksp
Some important factors that have an impact on the solubility product constant are:
- The common-ion effect (the presence of a common ion lowers the value of Ksp).
- The diverse-ion effect (if the ions of the solutes are uncommon, the value of Ksp will be high).
- The presence of ion-pairs.
Common Ion Effect
The common ion effect is an effect that suppresses the ionization of an electrolyte when another electrolyte (which contains an ion which is also present in the first electrolyte, i.e. a common ion) is added. It is considered to be a consequence of Le Chatlier’s principle (or the Equilibrium Law).
What is the Common Ion Effect?
The statement of the common ion effect can be written as follows – in a solution wherein there are several species associating with each other via a chemical equilibrium process, an increase in the concentration of one of the ions dissociated in the solution by the addition of another species containing the same ion will lead to an increase in the degree of association of ions.
An example of the common ion effect can be observed when gaseous hydrogen chloride is passed through a sodium chloride solution, leading to the precipitation of the NaCl due to the excess of chloride ions in the solution (brought on by the dissociation of HCl). This effect cannot be observed in the compounds of transition metals. This is because the d-block elements have a tendency to form complex ions. This can be observed in the compound cuprous chloride, which is insoluble in water. This compound can be dissolved in water by the addition of chloride ions leading to the formation of the CuCl2– complex ion, which is soluble in water.
This effect cannot be observed in the compounds of transition metals. This is because the d-block elements have a tendency to form complex ions. This can be observed in the compound cuprous chloride, which is insoluble in water. This compound can be dissolved in water by the addition of chloride ions leading to the formation of the CuCl2– complex ion, which is soluble in water.
Effect on Solubility
The way in which the solubility of a salt in a solution is affected by the addition of a common ion is discussed in this subsection.
- The common ion effect can be used to obtain drinking water from aquifers (underground layer of water mixed with permeable rocks or other unconsolidated materials) containing chalk or limestone. Sodium carbonate (chemical formula Na2CO3) is added to the water in order to decrease the hardness of the water.
- In the treatment of water, the common ion effect is used to precipitate out the calcium carbonate (which is sparingly soluble) from the water via the addition of sodium carbonate, which is highly soluble.
- A finely divided calcium carbonate precipitate of a very pure composition is obtained from this addition of sodium carbonate. The CaCO3 precipitate is, therefore, a valuable by-product which can be used in the process of manufacturing toothpaste.
- Since soaps are the sodium salts of carboxylic acids containing a long aliphatic chain (fatty acids), the common ion effect can be observed in the salting-out process which is used in the manufacturing of soaps. The soaps are precipitated out by adding sodium chloride to the soap solution in order to reduce its solubility.
However, it can be noted that water containing a respectable amount of Na+ ions, such as seawater and brackish water, can hinder the action of soaps by reducing their solubility and therefore their effectiveness.
pH and the Common-Ion Effect
When the conjugate ion of a buffer solution (solution containing a base and its conjugate acid, or acid and its conjugate base) is added to it, the pH of the buffer solution changes due to the common ion effect.
- An example of such an effect can be observed when acetic acid and sodium acetate are both dissolved in a given solution, generating acetate ions. However, sodium acetate completely dissociates but the acetic acid only partly ionizes. This is because acetic acid is a weak acid whereas sodium acetate is a strong electrolyte.
- As per Le Chatelier’s principle, the new acetate ions put forth by sodium acetate facilitate the suppression of the ionization of acetic acid, thereby shifting the equilibrium to the left. Since the dissociation of acetic acid is reduced, the pH of the solution is increased.
- Therefore, the common ion solution containing acetic acid and sodium acetate will have an increased pH and will, therefore, be less acidic when compared to an acetic acid solution.
Thus, the common ion effect, its effect on the solubility of a salt in a solution, and its effect on the pH of a solution.
|
127 videos|244 docs|87 tests
|
FAQs on Solubility Equilibria of Sparingly Soluble Salts - Chemistry Class 11 - NEET
| 1. What is solubility equilibrium? |  |
| 2. How does temperature affect the solubility equilibrium of sparingly soluble salts? |  |
| 3. What factors determine the solubility of sparingly soluble salts? |  |
| 4. How can we calculate the solubility product constant (Ksp) for sparingly soluble salts? |  |
| 5. What are some common applications of solubility equilibria for sparingly soluble salts? |  |

|
Explore Courses for NEET exam
|

|

















