Class 10 Science Chapter 1 Question Answers - Chemical Reactions and Equations
Q1: What is a Chemical Reaction?
Ans: A chemical reaction refers to the process that leads to the transformation of one set of chemical substances into another.
A Chemical ReactionIt involves the making or breaking of bonds between atoms, ions, or molecules. During a chemical reaction, reactants are transformed into products. For instance, when iron rusts, the iron (reactant) reacts with oxygen to form iron oxide (product).
Reaction: Rusting of Iron
Q2: What is a balanced chemical equation? Why is it necessary to balance a chemical equation?
Ans: A balanced chemical equation is one in which the number of atoms involved on the reactant side is equal to the number of atoms on the product side. This is based on the law of conservation of mass which states that matter cannot be created or destroyed. For example, the balanced chemical equation for the reaction between hydrogen and oxygen to form water is 2H2 + O2 → 2H2O.
A Balanced Chemical Equation
Need to Balance a Chemical Equation:
- Balancing a chemical equation is essential because it follows the Law of Conservation of Mass, which states that the total number of atoms of each element in the reactants must be equal to the total number of atoms of that element in the products.
- This law implies that the number of atoms for each element on the reactant side should be the same as the number of atoms for that element on the product side.
- Without balance, the chemical equation may incorrectly represent the actual reaction, leading to inaccurate predictions about the quantities of reactants needed or products formed.
Q3: What is the method of balancing chemical equations?
Ans: The hit-and-trial method is used to balance simple chemical equations. In this method, coefficients before the symbols/formulae of the reactants and products are adjusted in such a way that the total number of atoms of each element on both sides becomes equal.
For example, consider the unbalanced equation
H2 + O2 → H2O
Here, there are 2 H atoms on the left and only 1 on the right, and 2 O atoms on the left but only 1 on the right. By adjusting coefficients, we get the balanced equation as
2H2 + O2 → 2H2O
Now, there are 4 H atoms and 2 O atoms on both sides.
Q4: What are the characteristics of chemical reactions?
Ans: Characteristics of chemical reactions are as follows:
(i) Evolution of a gas
(ii) Formation of a precipitate
(iii) Change in color
(iv) Change in temperature
(v) Change in the state.
Q5: What is a Precipitation reaction? Give an example.
Ans: A precipitation reaction is a type of chemical reaction where two soluble salts in aqueous solution combine to form an insoluble salt (precipitate). This precipitate can be separated from the solution by filtration.
For example, when solutions of silver nitrate and sodium chloride are mixed, a white insoluble salt, silver chloride (AgCl), precipitates out of the solution:
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq).
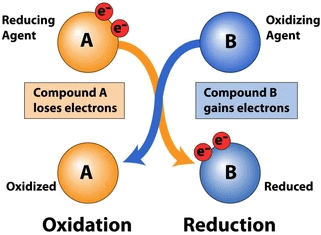
Q6: What is a redox reaction?
Ans: A redox (reduction-oxidation) reaction is type of chemical reaction that involves the transfer of electrons between two species. Redox reactions are of two types:
(i) Oxidation
(ii) Reduction
An oxidation reaction is one in which a substance loses electrons, while a reduction reaction is one in which a substance gains electrons. For example, in the reaction between hydrogen and oxygen to form water, hydrogen is oxidized (loses electrons) and oxygen is reduced (gains electrons): 2H2 + O2 → 2H2O.
Q7: In the equations given below, state giving reasons, for whether substances have been oxidized or reduced.
(i) PbO + CO → Pb + CO2
(ii) H2S + Cl2 → 2HCl + S
Ans:
(i) In this reaction, carbon monoxide (CO) is oxidized because it gains oxygen to form carbon dioxide (CO₂).
Lead oxide (PbO) is reduced to lead (Pb) because it loses oxygen. The reaction can be broken down into half-reactions to illustrate electron transfer:
Reduction (Gain of electrons):
PbO + 2e⁻ → Pb + O²⁻
(Lead oxide is reduced to lead)Oxidation (Loss of electrons):
CO + O²⁻ → CO₂ + 2e⁻
(Carbon monoxide is oxidized to carbon dioxide)
In this reaction:- PbO acts as the oxidizing agent because it accepts electrons, which leads to the oxidation of CO.
- CO acts as the reducing agent because it donates electrons, causing reduction of PbO.
(ii) In this reaction, hydrogen sulfide (H₂S) is oxidized to sulfur (S) because it loses hydrogen.
Chlorine (Cl₂) is reduced to hydrogen chloride (HCl) because it gains electrons. The half-reactions for this process are:
Oxidation (Loss of electrons):
H₂S → S + 2H⁺ + 2e⁻
(Hydrogen sulfide is oxidized to sulfur)Reduction (Gain of electrons):
Cl₂ + 2e⁻ → 2Cl⁻
(Chlorine is reduced to chloride ions, forming hydrochloric acid)In this reaction:
- Cl₂ acts as the oxidizing agent because it accepts electrons, causing oxidation of H₂S.
- H₂S acts as the reducing agent because it donates electrons, causing reduction of Cl₂.
Q8: What are the different ways that can make chemical equations more informative
Ans: Chemical equations can be made more informative by:-
(i) By indicating the physical states of the reactants and products. Example: Gaseous state is indicated by the symbol (g).
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)(ii) By indicating the heat changes that take place in the reaction. For example: An exothermic reaction is indicated by writing "Heat" or "Heat energy" or "Energy" on the products side of an equation.
C(s) + O2(g) → CO2(g) + Heat(iii) By indicating the “conditions” under which the reaction takes place.
Q9: A sample of water weed was placed in water and exposed to sunlight. Bubbles of gas are seen on the surface of the leaves.
(i) Name the gas evolved.
(ii) Name the process taking place.
(iii) Write a balanced equation of reaction taking place.
Ans:
(i) Oxygen
(ii) Photosynthesis
(iii) Carbon Dioxide + Water→Glucose + Oxygen
that means,
6CO2 + 6H2O → C6H12O6 + 6O2Explanation in detail:
- Photosynthesis is the process in which plants use carbon dioxide and water to form glucose in the presence of energy from the sunlight and release oxygen alongside the glucose molecule.
- 6CO2 + 6H2O → C6H12O6 + 6O2
- As the oxygen is evolved in the process, the bubbles of gas seen on the surface of leaves are of oxygen.
- Photosynthesis occurs in plants having chloroplasts.
Q10: Give balanced equations, wherever possible, or where this is not possible, explain the following by means of examples:
(i) A reaction which gives out heat.
(ii) A reaction that takes place with the help of sunlight.
(iii) A reaction that is brought about by the electric current.
(iv) A reversible reaction.
(v) A reaction with a solid and gas that produces heat.
Ans:
(i) Coke on heating in air, catches fire and liberates a large amount of heat.
C + O2 → CO2 + Heat
(ii) Carbon dioxide and water react in the presence of chlorophyll and sunlight to form glucose and oxygen.
6CO2 + 6H2O → C6H12O6 + 6O2
(iii) Molten lead bromide decomposes into lead metal and bromide on the passage of electric current.
PbBr2 → Pb + Br2
(iv) The reaction between red hot iron and steam is reversible.
3Fe + 4H2O ⇌ Fe3O4 + 4H2
(v) When magnesium burns in air or oxygen it liberates a large amount of heat.
2Mg + O2 → 2MgO + Heat.
Q11: Balance the following chemical equations:
(i) Mg + N2 → Mg3N2
(ii) KClO3 → KCI + O2
Ans:
(i) Mg + N2 → Mg3N2
Balance equation:- The number of atoms of each type in the reaction is the same on both reactants and the product sides is called a balance equation.
According to the question,
Consider the given equation as follows:
Mg + N2 → Mg3N2
Since the left-hand side is called the reactant side. The right-hand side is called the product side.
On the reactant side,
The number of atoms of Mg and n are 1 and 2 respectively.
On the product side,
The number of atoms of Mg and n are 3 and 2 respectively.
To balance the equation,
Multiply Mg by 3, we get
3Mg + N2 ⇒ Mg3N2
This implies that the number of atoms on both sides are equal.(ii) KClO3 → KCl + 3O2.
KClO3 → KCl + O2
Multiply oxygen by 3
KClO3 → KCl + 3O2
Multiply KClO3 by 2
2KClO3 → KCl + 3O2
Multiply KCl by 2
2KClO3 → 2KCl + 3O2
Hence, equation balanced
Q12: Write the uses of decomposition reactions.
Ans:
- Metal Extraction: They play a vital role in extracting metals like aluminum, sodium, and potassium from their compounds in ores through processes like electrolysis.
- Chemical Synthesis: Decomposition reactions are employed to create specific chemicals and compounds by breaking down complex substances.
- Environmental Applications: In waste treatment and pollution control, decomposition reactions help break down harmful substances into less toxic forms.
- Food Preparation: Baking soda (sodium bicarbonate) decomposes to release carbon dioxide, making dough rise in baking.
- Explosives: Decomposition reactions are involved in explosive devices, releasing energy rapidly.
Q13: What do you understand by the term corrosion?
Ans: Corrosion is when metals like iron, copper, or silver slowly get damaged because of things around them like water or acids. For example, when a new iron object is left outside, it starts to turn reddish-brown, which we call rust. This happens because the metal is reacting with air and water.Other metals like silver might turn black, and copper might turn green. These color changes show that the metal is getting damaged. Corrosion is a problem because it can ruin things like cars, bridges, and fences, making them weak and unsafe.
Q14: Can rancidity retard by storing foods away from the light?
Ans: This process is called rancidity. Rancidity is a process whereby food products, particularly those containing fats and oils, deteriorate in quality, leading to unpleasant smells and tastes. This occurs when the fats and oils within the food oxidize over time. If food is stored away from light, this oxidation process slows down, which retards the development of rancidity. Thus, proper storage can significantly delay the onset of this undesirable characteristic.
Example: Oil becomes rancid (rancid oil) because of the decomposition of fats it has, or sometimes milk becomes rancid due to bacterial growth, etc.
|
85 videos|437 docs|75 tests
|
FAQs on Class 10 Science Chapter 1 Question Answers - Chemical Reactions and Equations
| 1. What is a chemical reaction and how is it different from a physical change? |  |
| 2. What are the types of chemical reactions commonly studied in Class 10? |  |
| 3. How do you balance a chemical equation? |  |
| 4. What is the significance of the law of conservation of mass in chemical reactions? |  |
| 5. Can you provide an example of a simple chemical reaction and its balanced equation? |  |

|
Explore Courses for Class 10 exam
|

|

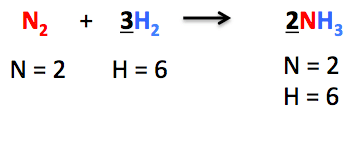


 Other metals like silver might turn black, and copper might turn green. These color changes show that the metal is getting damaged. Corrosion is a problem because it can ruin things like cars, bridges, and fences, making them weak and unsafe.
Other metals like silver might turn black, and copper might turn green. These color changes show that the metal is getting damaged. Corrosion is a problem because it can ruin things like cars, bridges, and fences, making them weak and unsafe.


















