JEE Advanced (Subjective Type Questions): Heat & Thermodynamics | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1.A sinker of weight w0 has an apparent weight w, when weighed in a liquid at a temperature t1 and w2 when weight in the same liquid at temperature t,. The coefficient of cubical expansion of the material of sinker is β. What is the coefficient of volume expansion of the liquid.
Ans.
Solution. 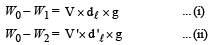
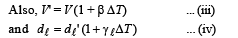
From (ii), (iii) and (iv)
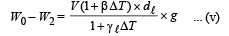
Dividing (i) and (v), we get
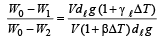
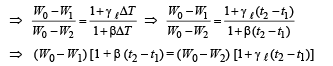
Q.2. Three rods of material X and three rods of material Y are connected as shown in the figure. All the rods are of identical length and cross-sectional area. If the end A is maintained at 60°C and the junction E at 10°C. Calculate the temperature of the junctions B, C and D. The thermal conductivity of X is 0.92 cal/sec-cm-°C and that of Y is 0.46 cal/sec-cm-°C.
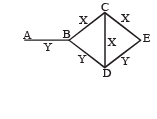
Ans. TB = 30°C, TC = TD = 20°C
Solution. KX = 0.92 cal/sec-cm-°C
KY = 0.46 cal/sec-cm-°C
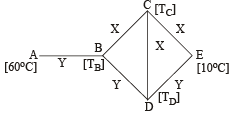
NOTE THIS STEP : The heat flow through AB is divided into two path BC and BD. Symmetry shows that no heat will flow through CD. Therefore
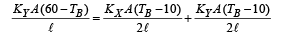
On solving the above equation, we get TB = 30°C
As C is a point at the middle of BE therefore temperature at C is 20°C.
Similarly temperature at D is also 20°C.
Q.3. Given samples of 1 c.c. of hydrogen and 1c.c. of oxygen, both at N.T.P. which sample has a larger number of molecules?
Ans. Same
Solution. PV = nRT
When P, T are same n∝V
As volumes are same, both samples will have equal number of molecules
Q.4. A Solid material is supplied with heat at a constant rate.
The temperature of the material is changing with the heat input as shown in the graph in figure. Study the graph carefully and answer the following questions :
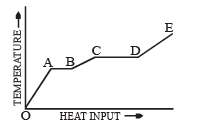
(i) What do the horizontal regions AB and CD represent?
(ii) If CD is equal to 2AB, what do you infer?
(iii) What does the slope of DE represent?
(iv) The slope of OA > the slope of BC. What does this indicate?
Solution. (i) Region AB : Heat is absorbed by the material at a constant temperature called the melting point.
The phase changes from solid to liquid.
Region CD : Heat is absorbed by the material at a constant temperature called the boiling point. The phase changes from liquid to gas.
(ii) Latent heat of vaporisation = 2 (latent heat of fusion)
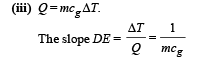
NOTE : The slope DE indicates that the temperature of the solid begins to rise.
(iv) The reciprocal of heat capacity in solid state is greater than the reciprocal of heat capacity in liquid state
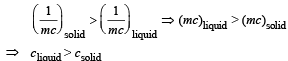
Q.5. A jar contains a gas and a few drops of water at T°K. The pressure in the jar is 830 mm of Hg. The temperature of the jar is reduced by 1%. The saturated vapour pressures of water at the two temperatures are 30 and 25 mm of Hg.
Calculate the new pressure in the jar.
Ans. 817 mmHg
Solution.
P1 = 830 – 30 = 800 mm Hg ; P2 ?
V1 = V ; V2 = V ; T1 = T ; T2 = T – 0.01 T = 0.99 T
∴ Total pressure in the jar = 792 + 25 = 817 mm Hg
Q.6. A cyclic process ABCA shown in the V-T diagram (fig) is performed with a constant mass of an ideal gas. Show the same process on a P-V diagram
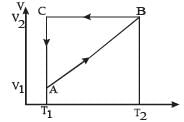
(In the figure, CA is parallel to the V-axis and BC is parallel to the T-axis)
Solution. A → B A straight line between A and B in V∝T graph indicates V ∝ T
⇒ Pressure is constant.
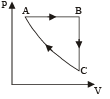
B → C Volume is constant. Since the temperature is decreasing, the pressure should also decrease.
C → A The temperature is constant but volume decreases.
The process is isothermal.
Q.7. A lead bullet just melts when stopped by an obstacle.
Assuming that 25 per cent of the heat is absorbed by the obstacle, find the velocity of the bullet if its initial temperature is 27°C. (Melting point of lead = 327°C, specific heat of lead = 0.03 calories /gm/°C, latent heat of fusion of lead = 6 calories / gm, J = 4.2 joules /calorie).
Ans. 12.96 m/s
Solution. Lead bullet just melts when stopped by an obstacle. Given that 25% of the heat is absorbed by the obstacle. Therefore 75% heat is used in melting of lead. Initial temp. = 27°C M.P. = 300°C
(0.75) K.E. = Heat utilised in increasing the temperature and heat utilised to melt lead at 300°C
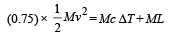
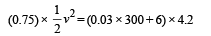
[4.2 to convert into S.I. system] v = 12.96 m/s
Q.8. Calculate the work done when one mole of a perfect gas is compressed adiabatically. The initial pressure and volume of the gas are 105 N/m2 and 6 litres respectively. The final volume of the gas is 2litre. Molar specific heat of the gas at constant volume is 3R/2.
Ans. –973.1 J
Solution. Work don in an adiabatic process is
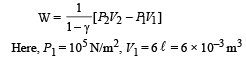
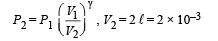
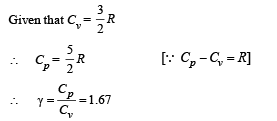
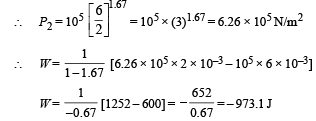
Work done is negative because the gas is compressed.
Q.9. A solid sphere of copper of radius R and a hollow sphere of the same material of inner radius r and outer radius A are heated to the same temperature and allowed to cool in the same environment. Which of them starts cooling faster ?
Ans. hollow sphere
Solution. NOTE : Since the temperature and surface area is same, therefore the energy emitted per second by both spheres is same.
We know that Q = mcΔT
Since Q is same and c is also same (both copper).
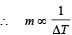
Mass of hollow sphere is less;
∴ Temperature change will be more.
∴ Hollow sphere will cool faster.
Q.10. One gram mole of oxygen at 27° and on e atmospheric pressure is enclosed in a vessel.
(i) Assuming the molecules to be moving with Vrms, Find the number of collisions per second which the molecules make with one square metre area of the vessel wall.
(ii) The vessel is next thermally insulated and moved with a constant speed Vo. It is then suddenly stopped. The process results in a rise of the temperature of the gas by 1°C. Calculate the speed Vo.
Ans. 1.97 × 1027, 35.6 m/s
Solution. 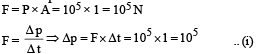
Now, momentum change per second (Δp) = n × 2mv ...(ii)
Where n is the number of collisions per second per square metre area From (i) and (ii)
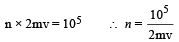
Root mean square velocity

According to mole concept 6.023 × 1023 molecules will have mass 32 g
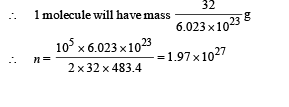
(ii) The kinetic energy of motion of molecules will be converted into heat energy.
K.E. of 1 gm mole of oxygen = 1/2 Mv20 ... (i)
where v0 is the velocity with which the vessel was moving.
The heat gained by 1 gm mole of molecules at constant volume for 1°C rise in temperature = nCvΔT = 1 × Cv × 1 = Cv ... (ii)
From (i) and (ii)
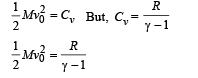
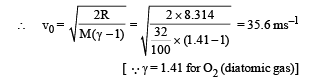
Q.11. The rectangular box shown in Fig has a partition which can slide without friction along the length of the box. Initially each of the two chambers of the box has one mole of a mono-atomic ideal gas (γ = 5/3) at a pressure P0, volume V0 and temperature T0. The chamber on the left is slowly heated by an electric heater. The walls of the box and the partition are thermally insulated. Heat loss through the lead wires of the heater is negligible. The gas in the left chamber expands pushing the partition until the final pressure in both chambers becomes 243 P0/32. Determine (i) the final temperature of the gas in each chamber and (ii) the work done by the gas in the right chamber.
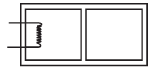
Ans. 12.9 T0, 2.25 T0, –15.58T0
Solution. For the left chamber
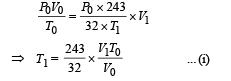
For the right chamber for adiabatic compression
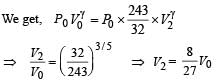
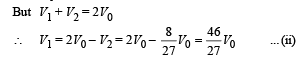
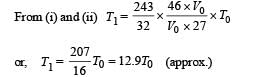
To find the temperature in the second chamber (right), we apply
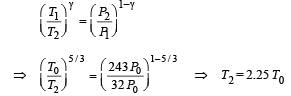
Work done in right chamber (adiabatic process)
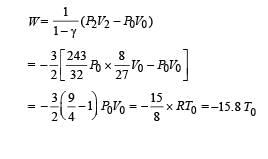
Q.12. Two glass bulbs of equal volume are connected by a narrow tube and are filled with a gas at 0°C and a pressure of 76 cm of mercury. One of the bulbs is then placed in melting ice and the other is placed in a water bath maintained at 62°C. What is the new value of the pressure inside the bulbs? The volume of the connecting tube is negligible.
Ans. 83.75 cm Hg
Solution. Let x moles shift from high temperature side to low temperature side.
for left bulb PV = nRT
76 × V = nR × 273 Initially
P' × V = (n + x) R × 273 Finally
Dividing, we get
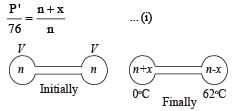
For right bulb
76 × V = nR × 273 Initially
P' × V = (n – x) R × 335 Finally .
On dividing,

From (i) and (ii)
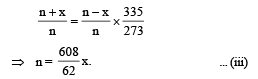
Substituting the value of (iii) in (i), we get
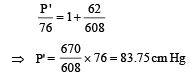
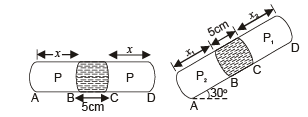
Q.13. A thin tube of uniform cross-section is sealed at both ends.
It lies horizontally, the middle 5 cm containing mercury and the two equal end containing air at the same pressure P.
When the tube is held at an angle of 60° with the vertical direction, the length of the air column above and below the mercury column are 46cm and 44.5 cm respectively. Calculate the pressure P in centimeters of mercury. (The temperature of the system is kept at 30°C).
Ans. 75.4 cm
Solution.
Let A be the area of cross-section of the tube.
Since temperature is the same, applying Boyle's law on the side AB
P × (x × A) = P2 × (x2 × A) ... (i)
Applying Boyle's law in section CD
P × (x × A) = P1 × (x1 × A) ... (ii)
From (i) and (ii)
P1 × (x1 × A) = P2 × (x2 × A)
⇒ P1x1 = P2x2
where P2 = P1 + Pressure due to mercury column
Pressure due to mercury column
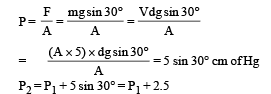
Substituting this value in (iii)
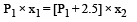
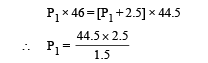
Substituting this value in (ii)
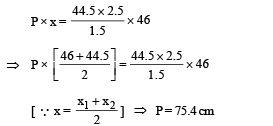
Q.14. An ideal gas has a specific heat at constant pressure CP= 5R/2. The gas is kept in a closed vessel of volume 0.0083 m3, at a temperature of 300 K and a pressure of 1.6 × 106 N/ m2. An amount of 2.49 × 104 Joules of heat energy is supplied to the gas. Calculate the final temperature and pressure of the gas.
Ans. 675 K, 3.6 × 106 N/m2
Solution. We know that PV = nRT
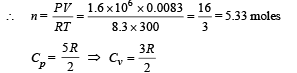
When 2.49 × 104 J of heat energy is supplied at constant volume then we can use the following relationship to find change in temperature.
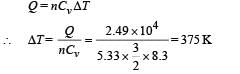
Therefore, the final temperature
= 300 + 375 = 675 K
Applying Gay Lussac's Law, to find pressure.
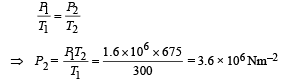
Q.15. Two moles of helium gas (γ = 5/3) are initially at temperature 27°C and occupy a volume of 20 litres. The gas is first expanded at constant pressure until the volume is doubled.
Then it undergoes an adiabatic change until the temperature returns to its initial value.
(i) Sketch the process on a p-V diagram. (ii) What are the final volume and pressure of the gas ? (iii) What is the work done by the gas ?
Ans. (ii) 113 l, 0.44 × 105 N/m2 (iii) 12450 J
Solution. (i) P – V diagram is drawn below.
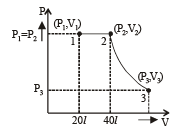
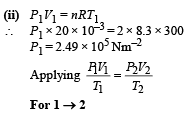
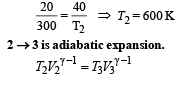
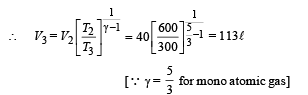
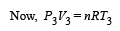
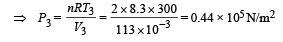
(NOTE : T3 = T1 given)
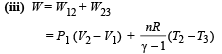
W12 = work done at constant pressure
W23 = work done in adiabatic condition
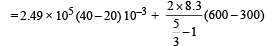
= 4980 + 7470 = 12450 J
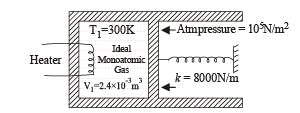
Q.16. An ideal monatomic gas is confined in a cylinder by a springloaded piston of cross-section 8.0 × 10–3 m2. Initially the gas is at 300 K and occupies a volume of 2.4 × 10–3 m3 and the spring is in its relaxed (unstretched, uncompressed) state, fig. The gas is heated by a small electric heater until the piston moves out slowly by 0.1 m. Calculate the final temperature of the gas and the heat supplied (in joules) by the heater. The force constant of the spring is 8000 N/m, atmospheric pressure is 1.0 × 105 Nm–2. The cylinder and the piston are thermally insulated. The piston is massless and there is no friction between the piston and the cylinder. Neglect heat loss through lead wires of the heater. The heat capacity of the heater coil is negligible. Assume the spring to be massless.
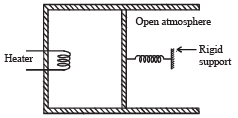
Ans. 800 K, 720 J
Solution. KEY CONCEPT : The final pressure on the gas = atm pressure + pressure due to compression of spring
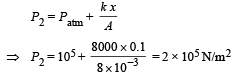
The final volume,
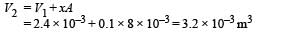
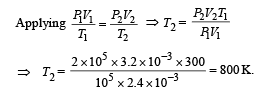
NOTE : Heat supplied by the heater is used for expansion of the gas, increasing its temperature and storing potential energy in the spring.
∴ Heat supplied
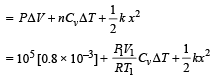
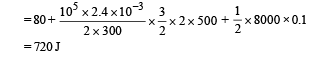
Q.17. An ideal gas h aving in itial pressure P, volume V and temperature T is allowed to expand adiabatically until its volume becomes 5.66 V while its temperature falls to T/2 . (i) How many degrees of freedom do the gas molecules have?
(ii) Obtain the work done by the gas during the expansion as a function of the initial pressure P and volume V.
Ans. (i) 5 (ii) 1.25 PV
Solution. (i) Let pressure = P, Volume = V and Temperature = T be the initial quantities and Pressure = P', Volume = 5.66 V Temperature = T/2 be the final quantities.
For adiabatic process
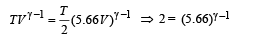
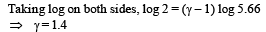
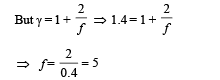
Thus degrees of freedom of gas molecules = 5
(ii) For adiabatic process the pressure-volume relationship is
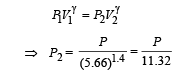
Work done for adiabatic process
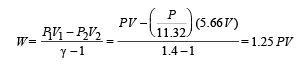
Q.18. Three moles of an ideal gas 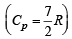 at pressure, PA and temperature TA is isothermally expanded to twice its initial volume. It is then compressed at constant pressure to its original volume. Finally gas is compressed at constant volume to its original pressure PA.
at pressure, PA and temperature TA is isothermally expanded to twice its initial volume. It is then compressed at constant pressure to its original volume. Finally gas is compressed at constant volume to its original pressure PA.
(a) Sketch P - V and P - T diagrams for the complete process. (b) Calculate the net work done by the gas, and net heat supplied to the gas during the complete process.
Ans. (b) 0.58 RTA, 0.58 RTA
Solution. (a) Process A to B (isothermal expansion)
PAVA = PBVB
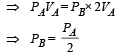
Process B to C (isobaric compression)
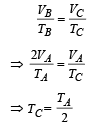
Process C to A [volume is constant]
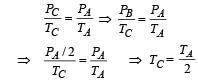
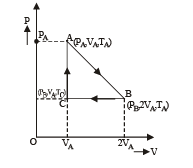
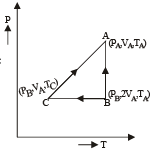
Let the system initially be at point A at pressure PA and temp TA and volume VA.
Process A to B
The system is isothermally expanded and reaches a new state B (PB, 2VA, TA) as shown in the figure.
Process B to C
The system is the compressed at constant pressure to its original volume to reach at state C (PB, VA, TC)
Process C to A Finally at constant volume, the pressure is increased to its original pressure to reach the state A again.
(b) The total work done
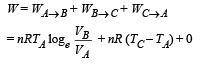
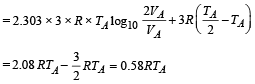
NOTE : The total work done is equal to the heat exchanged as the process is cyclic.
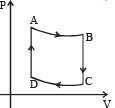
Q.19. Two moles of helium gas undergo a cyclic process as shown in Fig. Assuming the gas to be ideal, calculate the following quantities in this process
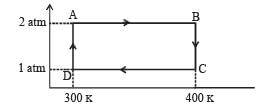
(a) The net change in the heat energy (b) The net work done (c) The net change in internal energy
Ans. (a) 1153 J (b) 1153J (c) Zero
Solution. Let us find out the work done in the cycle
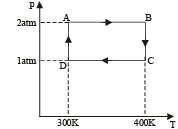
Work done from A to B (Isobaric process)
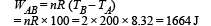
Work done from B to C (Isothermal process)
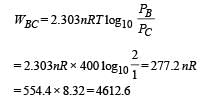
Work done from C to D (Isobaric process)
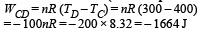
Work done from D to A (Isothermal process)
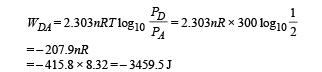
Q.20. One mole of a mono atomic ideal gas is taken through the cycle shown in Fig:
A → B : adiabatic expansion
B → C : cooling at constant volume
C → D : adiabatic compression
D → A : heating at constant volume
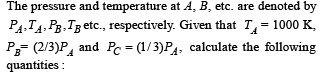
(i) The work done by the gas in the process A → B
(ii) The heat lost by the gas in the process B → C.
(iii) The temperature TD. [Given : (2 / 3)2/5 = 0.85]
Ans. (i) 1870 J (ii) –5298 J (iii) 500 K
Solution. Given TA = 1000 K
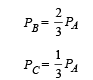
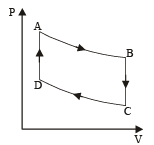
(i) WAB (adiabatic expansion)
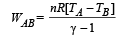
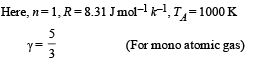
To find TB, we use
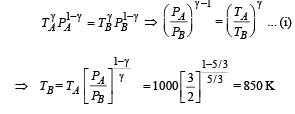
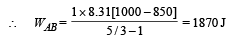
(ii) Heat Lost B → C
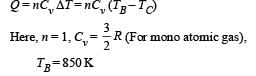
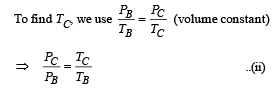
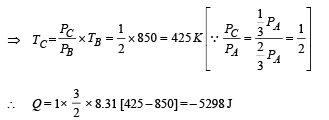
(iii) Temperature TD : C to D is adiabatic compression
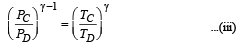

Multiplying (i) and (iii)
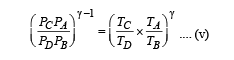
Multiplying (ii) and (iv)
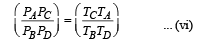
From (v) and (vi)
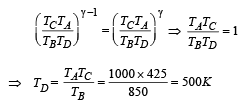
Q.21. An ideal gas is taken through a cyclic thermodynamic process through four steps. The amounts of heat involved in these steps are Q1 = 5960 J, Q2 = –5585 J, Q3 = –2980J and Q4 = 3645J, respectively. The correspending quantities of work involved are W1 = 2200 J, W2 = – 825 J,W3 = – 1100 J and W4 respectively.
1. Find the value of W4.
2. What is the efficiency of the cycle
Ans. (i) 765 J (ii) 10.82%
Solution. (i) The process is cyclic, therefore ΔU = 0
Now, ΔQ = ΔU + ΔW
⇒ΔQ =ΔW
⇒ Q1 + Q2 + Q3 + Q4 = W1 + W, + W3 + W4
⇒ 5960 – 5585 – 2980 + 3645 = 2200 – 825 – 1100 + W4
⇒ W4 = 765 J
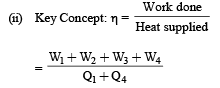
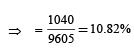
Q.22. A closed container of volume 0.02 m 3 contains a mixture of neon and argon gases, at a temperature of 27°C and pressure of 1 × 105 Nm–2. The total mass of the mixture is 28 g. If the molar masses of neon and argon are 20 and 40 g mol–1 respectively, find the masses of the individual gases in the container assuming them to be ideal (Universal gas constant R = 8.314 J/mol – K).
Ans. Mass of Neon = 4gm, mass of Argon = 24gm
Solution. The total pressure exerted by the mixture P = 105 Nm–2 Temperature T = 300 K ; Volume = 0.02 m3 Let there be x gram of Ne. Then mass of Ar will be 28 – x.

Partial pressure due to Neon;
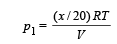
Partial pressure due to Argon
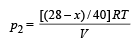
But according to Dalton's law of partial pressure
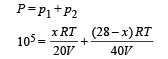
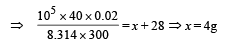
⇒ Mass of Neon = 4g
∴ Mass of Argon = 24g
Q.23. A gaseous mixture enclosed in a vessel of volume V consists of one mole of a gas A with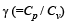 = 5 / 3 and another gas B with γ = 7/5 at a certain temperature T. The relative molar masses of the gases A and B are 4 and 32, respectively.The gases A and B do not react with each other and are assumed to be ideal. The gaseous mixture follows the equation PV19/13 = constant, in adiabatic processes.
= 5 / 3 and another gas B with γ = 7/5 at a certain temperature T. The relative molar masses of the gases A and B are 4 and 32, respectively.The gases A and B do not react with each other and are assumed to be ideal. The gaseous mixture follows the equation PV19/13 = constant, in adiabatic processes.
(a) Find the number of moles of the gas B in the gaseous mixture.
(b) Compute the speed of sound in the gaseous mixture at T = 300 K.
(c) If T is raised by 1K from 300 K, find the % change in the speed of sound in the gaseous mixture.
(d) The mixture is compressed adiabatically to 1/5 of its initial volume V. Find the change in its adiabatic compressibility in terms of the given quantities.
Ans. (a) 2 mole (b) 400.03 m/s (c) 1/6 (d) -8.27´10-5V
Solution.
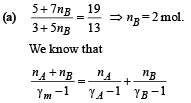
where γm = Ratio of specific heats of mixture
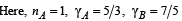
According to the relationship

(b) On substituting the values we get nB = 2 mol.
We know that velocity of sound in air is given by the relationship
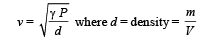
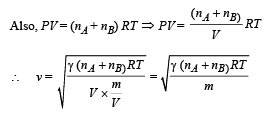
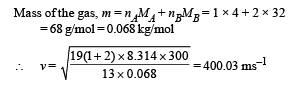
(c) Velocity of sound,
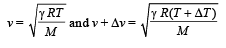
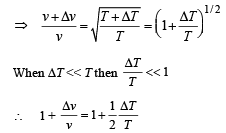
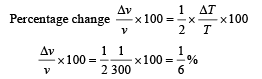
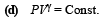
Differentiating the above equation
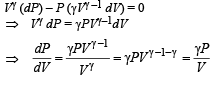

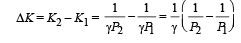
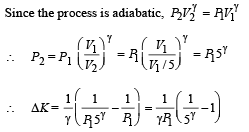
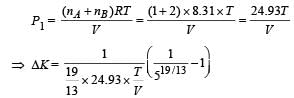
= – 8.27 × 10–5 V [∴ T = 300 K]
Q.24. At 27°C two moles of an ideal monoatomic gas occupy a volume V. The gas expands adiabaticallly to a volume 2V. Calculate (i) the final temperature of the gas, (ii) change in its internal energy, and (iii) the work done by the gas during this process.
Ans. (i) 189 K (ii) –2767 J (iii) 2767 J
Solution. 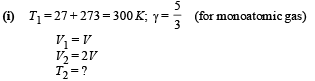
Since the gas expands adiabatically.
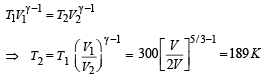
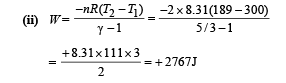
Change in internal Energy According to first law of thermodynamics
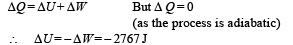
(iii) W = 2767 J
Q.25. The temperature of 100g of water is to be raised from 24°C to 90°C by adding steam to it. Calculate the mass of the steam required for this purpose.
Ans. 0.0122 Kg
Solution. Heat lost by steam = Heat gained by water
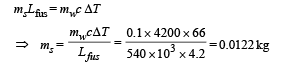
Q.26. One mole of a diatomic ideal gas (g = 1.4) is taken through a cyclic process starting from point A. The process A ⇒ B is an adiabatic compression, B ⇒ C is isobaric expansion, C ⇒ D is an adiabatic expansion, and D ⇒ A is isochoric. The volume ratios are VA / VB = 16 and VC / VB = 2 and the temperature at A is TA = 300 K.Calculate the temperature of the gas at the points B and D and find the efficiency of the cycle.
Ans. TB = 909 K, TD = 791 K, 61.4%
Solution. n = 1, for diatomic gas,
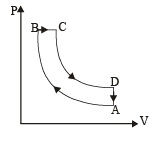
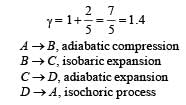
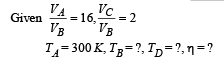
For adiabatic compression process A ⇒ B
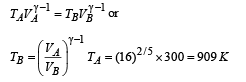
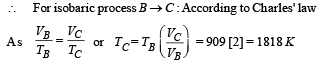
For adiabatic expansion process C ⇒ D :
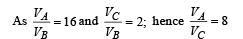
According to Poisson's law,
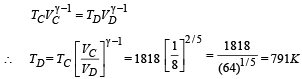


(∴ No heat is exchanged in adiabatic processes).
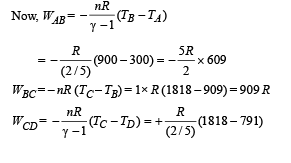
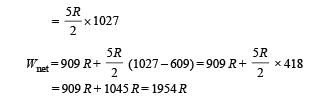
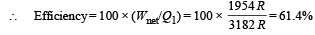
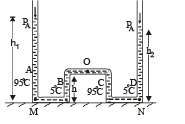
Q.27. The apparatus shown in the figure consists of four glass columns connected by horizontal sections. The height of two central columns B and C are 49 cm each. The two outer columns A and D are open to the atmosphere. A and C are maintained at a temperature of 95° C while the columns B and D are maintained at 5°C. The height of the liquid in A and D measured from the base the are 52.8 cm and 51cm respectively. Determine the coefficient of thermal expansion of the liquid.
Ans. 6.67 × 10–5 per °C
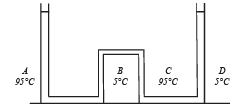
Solution. Let the pressure at point O be P0. Since the liquid is at equilibrium at M
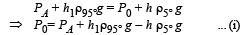
Since the liquid is at equilibrium at N
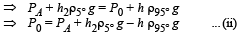
From (i) and (ii)
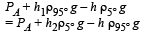
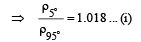
We know that
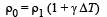
Applying the above formula, we get
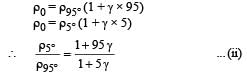
From (i) and (ii)
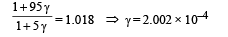
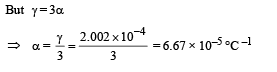
Q.28. One mole of an ideal monatomic gas is taken round the cyclic process ABCA as shown in Figure. Calculate
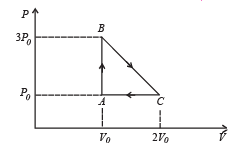
(a) the work done by the gas. (b) the heat rejected by the gas in the path CA and the heat absorbed by the gas in the path AB; (c) the net heat absorbed by the gas in the path BC; (d) the maximum temperature attained by the gas during the cycle.
Ans.
Solution. n = 1, For monoatomic gas :
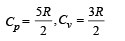
Cyclic process
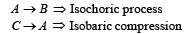
(a) Work done = Area of closed curve ABCA during cyclic process. i.e. ΔABC
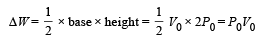
(b) Heat rejected by the gas in the path CA during isobaric compression process
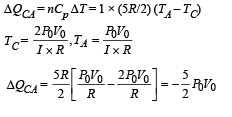
Heat absorbed by the gas on the path AB during isochoric process
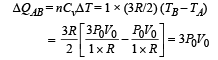
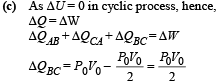
NOTE : As net heat is absorbed by the gas during path BC, temp. will reach maximum between B and C.
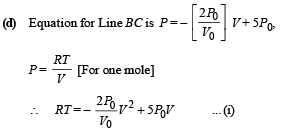
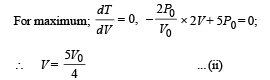
Hence from equation (i) and (ii)
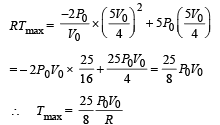
Q.29. A solid body X of heat capacity C is kept in an atmosphere whose temperature is TA = 300 K. At time t = 0 the temperature of X is T0 = 400 K. It cools according to Newton’s law of cooling. At time t1, its temperature is found to be 350 K.
At this time (t,), the body X is connected to a large box Y at atmospheric temperature TA, through a conducting rod of length L, cross-sectional area A and thermal conductivity K. The heat capacity of Y is so large that any variation in its temperature may be neglected. The cross-sectional area A of the connecting rod is small compared to the surface area of X. Find the temperature of X at time t = 3t1.
Ans.
Solution. Case (i) According to Newton's law of cooling
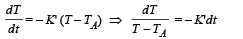
On integrating, we get
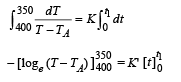
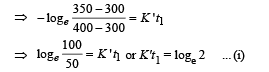
Case (ii)
NOTE : When the body X is connected to a large box Y. In this case cooling occurs by Newton's law of cooling as well as by conduction
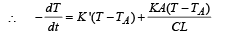
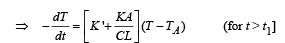
Where K = coefficient of thermal conductivity of the rod.
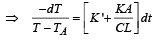
On integrating, we get
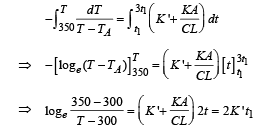
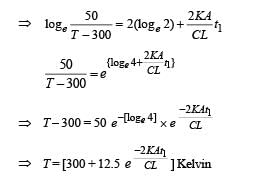
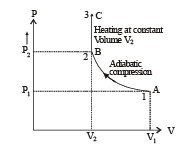
Q.30. Two moles of an ideal monatomic gas, initially at pressure p, and volume V1, undergo an adiabatic compression until its volume is V2. Then the gas is given heat Q at constant volume V2.
(a) Sketch the complete process on a p – V diagram.
(b) Find the total work done by the gas, the total change in its internal energy and the final temperature of the gas. [Give your answer in terms of p1, V1, V2, Q and R]
Ans.
Solution. n = no. of moles = 2,
(A) The complete process is shown on P-V diagram in the figure.
(B) (i) Total work done
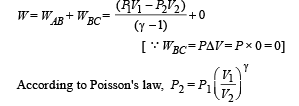
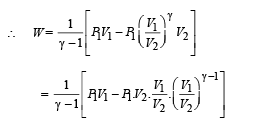
For monoatomic gas,
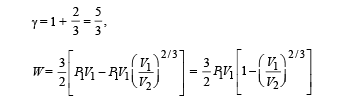
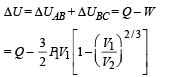
[according to first law of thermodynamics]
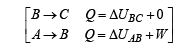
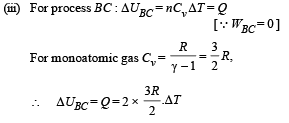
According to Poission's Law :
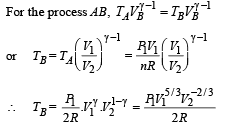
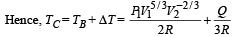
Q.31.Two moles of an ideal monatomic gas is taken through a cycle ABCA as shown in the P-T diagram. During the process AB, pressure and temperature of the gas vary such that PT = Constant. If T1 = 300 K, calculate
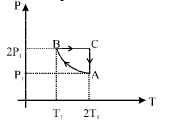
(a) the work done on the gas in the process AB and (b) the heat absorbed or released by the gas in each of the processes.
Give answer in terms of the gas constant R.
Ans. (a) 1200R (b) –2100R, 831.6 R
Solution. For PVx = Constt., Molar heat capacity
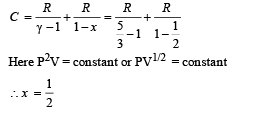
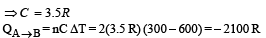
Process B – C: Process is isobaric therefore
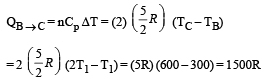
Heat is absorbed
Process C – A: Process is isothermal
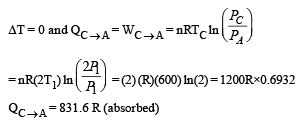
Q.32. An ice cube of mass 0.1 kg at 0oC is placed in an isolated container which is at 227oC. The specific heat S of the container varies with temperature T according to the empirical relation S = A + BT, where A = 100 cal/kg-K and B = 2 ´ 10-2 cal/kg-K2. If the final temperature of the container is 27oC, determine the mass of the container. (Latent heat of fusion of water = 8 *104 cal/kg, Specific heat of water = 103 cal/kg-K).
Ans. 0.495 Kg
Solution.
Here the equilibrium temperature is 273 + 27 = 300 K Also according to the principle of calorimetry Heat lost by container = Heat gained by ice.
Heat lost by container : NOTE : Since specific heat is variable, we need to take the help of calculus to find the heat lost by the container.
Let dQ be the heat lost when the temperature decreases by dT at any instant when the temperature of the container is T.
∴ dQ = mc dT
where m is the mass of the container and C = A + BT is specific heat at that temperature
∴ dQ = m (A + BT) dT
On integrating, we get
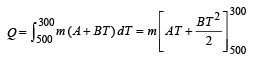
= – 21600 m calorie (heat lost)
Heat gained by ice This heat is to be divided into two parts
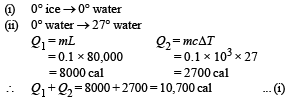
Heat lost = heat gained
21600 m = 10,700
⇒ m = 0.495 kg
Q.33. A monoatomic ideal gas of two moles is taken through a cyclic process starting from A as shown in figure. The volume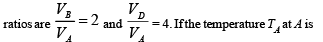 27oC.
27oC.
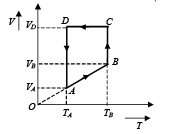
Calculate, (a) the temperature of the gas at point B, (b) heat absorbed or released by the gas in each process, (c) the total work done by the gas during the complete cycle.
Express your answer in terms of the gas constant R.
Ans. (a) 600 K (b) 1500R, 831.8R, –900R, –831.8R (c) 600R
Solution.
(a) Since AB is a straight line in V-T graph

(b) (i) A to B is a isobaric process
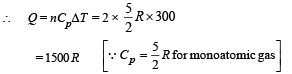
NOTE : Heat is absorbed as Q is positive. (ii) B to C is an isothermal process.
Since the temperature is not changing
∴ Internal energy change dU = 0
∴ From first law of thermodynamics dQ = dW

NOTE : Heat is absorbed since temperature is same but volume increases.
(iii) C To D is a isochoric process
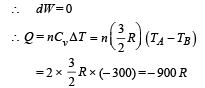
Volume is constant as heat is released.
(iv) D to A is an isothermal process
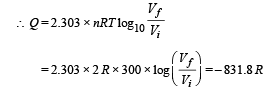
Heat is released as Q is positive.
(c) Total work done
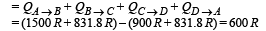
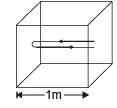
Q.34. A cubical box of side 1 meter contains helium gas (atomic weight 4) at a pressure of 100 N/m2. During an observation time of 1 second, an atom travelling with the root-meansquare speed parallel to one of the edges of the cube, was found to make 500 hits with a particular wall, without any collision with other atoms. Take R = 25/3 J/mol-K and k = 1.38 × 10-23 J/K
(a) Evaluate the temperature of the gas. (b) Evaluate the average kinetic energy per atom. (c) Evaluate the total mass of helium gas in the box.
Ans. (a) 160K (b) 3.312 × 10–21 J (c) 0.3012 gm
Solution. The distance travelled by an atom of helium in 1/500sec (time between two successive collision) is 2m. Therefore root mean square speed
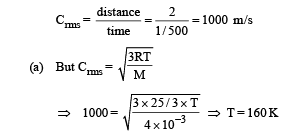
(b) Average kinetic energy of an atom of a monoatomic
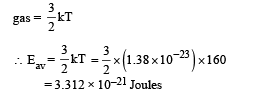
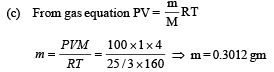
Q.35. An insulated container containing monoatomic gas of molar mass m is moving with a velocity v0. If the container is suddenly stopped, find the change in temperature.
Ans. mv20/3R
Solution. When container is stopped, velocity decreases by v0.
Therefore change in kinetic energy = 1/2(nm)v20 ... (i)
Here n = number of moles of gas present in the container.
The kinetic energy at a given temperature for a monatomic
gas is = 3/2 *nRT
∴ Change in kinetic energy =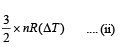
where ΔT = Change in temperature From (i) and (ii)
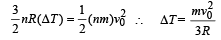
Q.36.
Hot oil is circulated through an insulated container with a wooden lid at the top whose conductivity
K = 0.149 J/(m-ºC-sec), thickness t = 5 mm, emissivity = 0.6.
Temperature of the top of the lid is maintained at Tl = 127ºC.
If the ambient temperature Ta = 27ºC.
Calculate : (a) rate of heat loss per unit area due to radiation from the lid. (b) temperature of the oil.
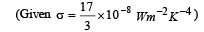
Ans. (a) 595 W/m2 (b) 419.83 K
Solution. (a) The rate of heat loss per unit area per second due to radiation is given by Stefan's-Boltzmann law
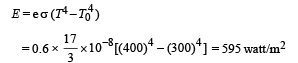
(b) Let Toil be the temperature of the oil.
Then rate of heat flow through conduction = Rate of heat flow through radiation
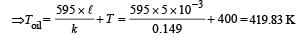
Q.37. A diatomic gas is enclosed in a vessel fitted with massless movable piston. Area of cross section of vessel is 1 m2.Initial height of the piston is 1 m (see the figure). The initial temperature of the gas is 300 K. The temperature of the gas is increased to 400 K, keeping pressure constant, calculate the new height of the piston. The piston is brought to its initial position with no heat exchange. Calculate the final temperature of the gas. You can leave answer in fraction. 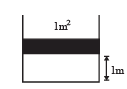
Ans.
Solution.At constant pressure, we have 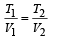
also, V = A × h
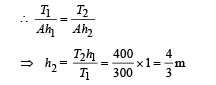
when the gas is compressed without heat exchange, the process is adiabatic
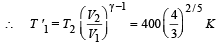
Q.38. A small spherical body of radius r is falling under gravity in a viscous medium. Due to friction the medium gets heated.
How does the rate of heating depends on radius of body when it attains terminal velocity?
Ans.Rate of heat produced ∝ r 5
Solution. Rate of heat produced = F × v
= (6 π η r v) v [∴ Viscous F =6 π η r v].
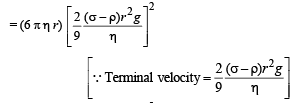
⇒ Rate of heat produced ∝ r 5
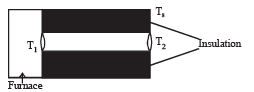
Q.39. A cylindrical rod of length l, thermal conductivity K and area of cross section A has one end in the furnace at temperature T1 and the other end in surrounding at temperature T2. Surface of the rod exposed to the surrounding has emissivity e. Also 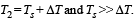
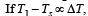 find the proportionality constant.
find the proportionality constant.
Ans.
Solution.
From the figure it is clear that emission takes place from the surface at temperature T2 (circular cross section). Heat conduction and radiation through lateral surface is zero.
Heat conducted through rod is
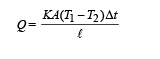
Energy emitted by surface of rod in same time Δt, is
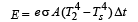
Since rod is at thermal equilibrium therefore E = Q
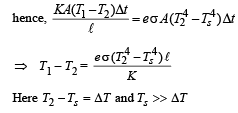
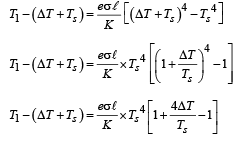
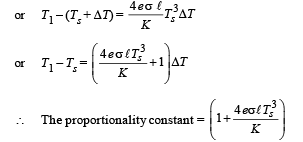
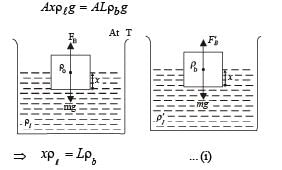
Q.40.A cubical block of co-efficient of linear expansion as is submerged partially inside a liquid of co-efficient of volume expension γℓ. On increasing the temperature of the system by ΔT,the height of the cube inside the liquid remains unchanged. Find the relation between αs and γℓ.
Ans. γ = 2α
Solution. Initially, at temperature T FB = mg
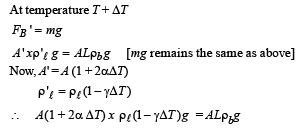
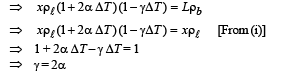
Q.41. A cylinder of mass 1 kg is given heat of 20,000J at atmospheric pressure. If initially the temperature of cylinder is 20°C, find
(a) final temperature of the cylinder. (b) work done by the cylinder. (c) change in internal energy of the cylinder
(Given that specific heat of cylinder = 400 J kg–1 °C–1, coefficient of volume expansion = 9 × 10–5 °C–1, Atmospheric pressure = 105 N/m2 and Density of cylinder = 9000 kg/m3)
Ans. 69.99°C, 0.0499J, 19999.95 J
Solution. (a) Heat supplied to the cylinder = Energy used to raise the temperature of cylinder + Energy used for work done by the cylinder.
Energy used to raise the temperature = mcΔT = 1 × 400 × (T – 20) ... (i)
where T°C is the final temperature of the cylinder.
Energy used for work done = Patm (V2– V1) = 105 (V2 – V1) ... (ii)
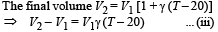
From (ii) and (iii),
Energy used for work done 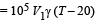
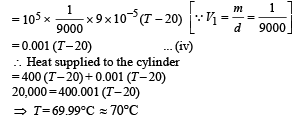
(b) Work done = 0.001 (69.99 – 20) = 0.0499 J
(c) Change in internal energy = 20,000 – 0.0499 = 19999.95 J.
Q.42. 0.05 kg steam at 373 K and 0.45 kg of ice at 253K are mixed in an insulated vessel. Find the equilibrium temperature of the mixture. Given, Lfusion = 80 cal/g = 336 J/g, Lvaporization = 540 cal/g = 2268 J/g, Sice = 2100 J/Kg K = 0.5 cal/gK and Swater = 4200 J/Kg K = 1 cal/gK
Ans. 273K or 0°C
Solution.
(1) Heat lost by steam at 100°C to change to 100°C water mLvap = 0.05 × 2268 × 1000 = 1,13,400 J
(2) Heat lost by 100°C water to change to 0°C water = 0.05 × 4200 × 100 = 21,000 J
(3) Heat required by 0.45 kg of ice to change its temperature from 253 K to 273 K = m × Sice × ΔT = 0.45 × 2100 × 20 = 18,900 J
(4) Heat required by 0.45 kg ice at 273 K to convert into 0.45 kg water at 273 K = mLfusion = 0.45 × 336 × 1000 = 151,200 J
From the above data it is clear that the amount of heat required by 0.45 kg of ice at 253 K to convert into 0.45 kg of water at 273 K (1,70,100 J) cannot be provided by heat lost by 0.05 kg of steam at 373 K to convert into water at 273 K.
Therefore the final temperature will be 273 K or 0°C.
|
446 docs|930 tests
|
















