Class 10 Exam > Class 10 Notes > Science Class 10 > Mindmap: Chemical Reactions & Equations
Mindmap: Chemical Reactions & Equations | Science Class 10 PDF Download
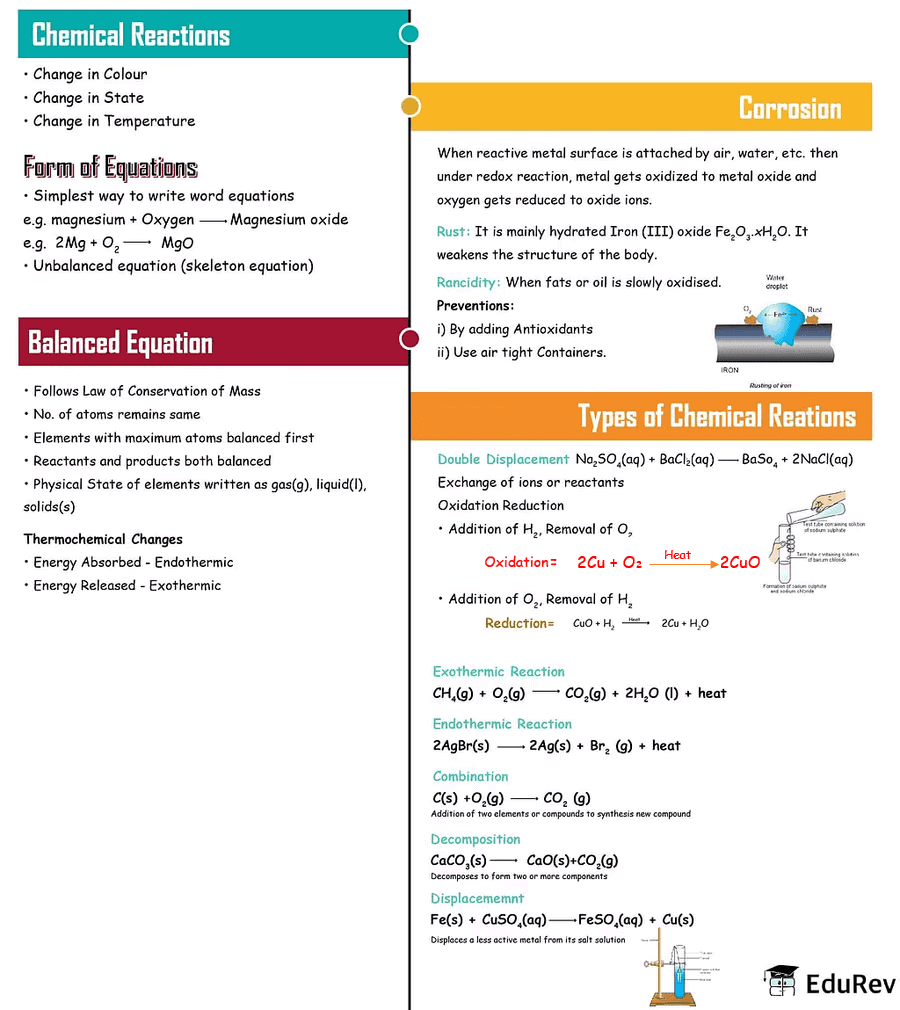
The document Mindmap: Chemical Reactions & Equations | Science Class 10 is a part of the Class 10 Course Science Class 10.
All you need of Class 10 at this link: Class 10
|
80 videos|512 docs|74 tests
|
FAQs on Mindmap: Chemical Reactions & Equations - Science Class 10
| 1. What are the different types of chemical reactions? |  |
| 2. How do you balance a chemical equation? |  |
Ans. To balance a chemical equation, you need to ensure that the number of atoms for each element is the same on both sides of the equation. This is done by adjusting the coefficients in front of the compounds. Start with the most complex molecule, and work your way to the simpler ones, adjusting coefficients as necessary.
| 3. What is the importance of chemical equations in chemistry? |  |
Ans. Chemical equations are essential in chemistry because they provide a concise way to represent chemical reactions. They show the reactants and products, indicating the conservation of mass, and allow chemists to calculate the amounts of substances involved in reactions, which is crucial for understanding reaction mechanisms and yields.
| 4. What are the signs that a chemical reaction has occurred? |  |
Ans. Signs that a chemical reaction has occurred include a change in color, the formation of a precipitate, the evolution of gas (bubbles), temperature change (either absorption or release of heat), and a change in odor. These indicators help in identifying that a chemical change has taken place.
| 5. Can you explain the difference between endothermic and exothermic reactions? |  |
Ans. Endothermic reactions absorb energy from their surroundings, resulting in a decrease in temperature in the immediate environment, while exothermic reactions release energy, usually in the form of heat, leading to an increase in temperature. Understanding these differences is crucial for studying energy changes in chemical processes.
Related Searches
















