Grade 8 Exam > Grade 8 Notes > Science for Grade 8 > NCERT Summary: Materials - Metals & Non-metal
Materials - Metals & Non-metal Summary Class 8 NCERT Summary
Introduction
- Materials are classified in two types i.e. metals and non-metals.
- Metals can be distinguished from non-metals on the basis of their physical and chemical properties.
Metals
Physical properties of metals
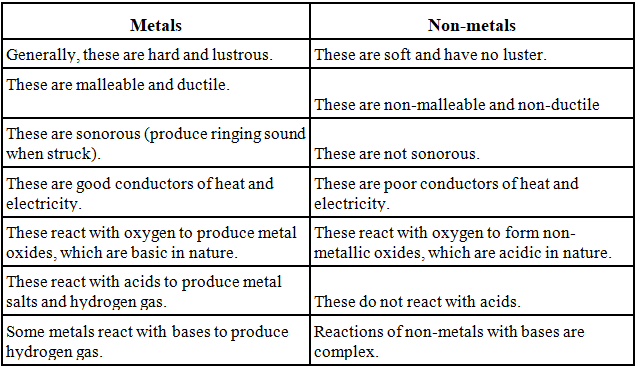
- They have shining surface in their pure state. It is also called metallic lustre.
- They are generally hard. The hardness varies from metal to metal.
- Metals are malleable. This means it can be made thin sheets by beating.
- Ductile which means it can be drawn into thin wires. As an example, Gold is highly ductile metal.
- The are good conductors of heat and have high melting point.
- The conduct electricity.
- Most of metals are sonorous which means it can produce sound.
Chemical Properties of metals
- Metals combine with oxygen to form metal oxides.
2Cu + O2 → 2CuO
4Al + 3O2 → 2Al2O3 - Most metal oxides are insoluble in water.
If metal oxides are soluble, they form alkali.
Na2O + H2O → 2NaOH
K2O + H2O → 2KOH - Sodium, potassium react very easily with O2. So, they are kept immersed in kerosene.
- Mg, Al, Zn, Pb form thin layers of oxides when kept in open.
Reaction of metals with water
- Metals produce metal oxide +H2 on reaction with water.
If oxide is soluble, then metal hydroxide is formed. - Magnesium doesn't react with cold water.
- Al, Zn, Fe do not react with H2O, but react with steam.
2Al + 3H2O → Al2O3 + 3H2
3Fe + 4H2O → Fe3O4 + 4H2
Non-metals
Physical properties of non-metals
- Non-metals are found in all the three states i.e. solid, liquid and gas, at room temperature.
- Iodine (non-metal) has lustre.
- Carbon has allotropes which means it exists in different forms.
- Diamond is hard and Graphite conducts electricity.
Difference between Metals and Non-metals
Reaction with Acids
- Metal + Dilute acid → Metal salt + H2
- H2 doesn’t evolve in the case of HNO3 as it is a strong oxidising agent. It oxidises H2.
- Cu does not react with acids like dilute H2SO4 and dilute HCl.
Aqua regia
- Freshly-prepared concentrated HCl and concentrated HNO3 in 3:1 ratio
- It can dissolve even gold and platinum.
Reaction with Bases
- Metals react with bases to produce hydrogen gas.
- Reactions of non-metals with bases are complex.
Uses of metals
- In making machinery, automobiles, jewellery, trains, aeroplanes, cooking utensils, etc.
- Gold is used for making jewellery, wires, and coins and in dentistry.
- Silver is used for making coins, ornaments, very thin wires, table cutlery and in photographic films.
- Copper is used for making wires, utensils, statues, alloys and coins.
- Iron is used for construction of ships, buildings, automobiles and railway bridges etc.
- Tin is used for tinning food cans, and making alloys.
- Lead is used for making batteries, and alloys.
- Zinc is used in prevention of rusting, making brass and bronze and in dry cells.
- Aluminium is used in making wires, foils, and alloys.
- Mercury is used for making amalgams and in thermometers.
- Magnesium is used for making fire works, and alloys.
Uses of non-metals
- They are used in fertilizers, in water purification process, crackers, etc. Oxygen, a non-metal, is essential for our life as all living beings inhale it during breathing.
- Nitrogen dilutes the activity of oxygen in air. It is used by plants to manufacture proteins.
- Oxygen is essential for respiration and combustion of fuels.
- Chlorine is used for bleaching fabrics, sterilization of drinking water, and in manufacturing insecticides and pesticides.
- Iodine is essential for proper functioning of human body, and in photographic films.
- Graphite is used as pencil lead, dry lubricant, in electrolytic cells and nuclear reactors.
- Helium is a noble gas which is used in weather observation balloons.
- Argon is a noble gas which is used for filling electric bulbs.
Types of Reactions
- Displacement reactions
In this reactions, a more reactive metal displaces a less reactive metal from their compounds in aqueous solutions. (However, a less reactive metal cannot displace a more reactive metal.)
Example:
CuSO4 (Blue) + Zn → ZnSO4 (Colourless) + Cu (Red)
(Copper Sulphate + Zinc → Zinc Sulphate + Copper)
Pb + CuCl2 → PbCl2 + Cu - Double displacement reaction
In this reaction, exchange of ions occurs between two compounds.
Na2SO4 + BaCl2 → BaSO4 + 2NaCl
Alloys
- Alloys are homogeneous mixtures of two or more metals (or metal and non-metal). Alloying is done to enhance the properties of metals.
- Alloys of aluminium are also useful as they are both light and strong. Some of its alloys are duralumin, magnelium, etc.
- Some alloys of iron are steel, stainless steel, etc. Steel is an alloy of iron and carbon.
- Some alloys of zinc are brass, bronze, and German silver.
- On the basis of composition, alloys are of two types: Substitutional alloys in which atoms of one element randomly replace the atoms of another metal. And interstitial alloys in which small atoms like hydrogen, boron, carbon and nitrogen occupy the holes in the crystal structure of the metal.
- On the basis of constituent elements, alloys are of two types:
- Ferrous alloys: They contain iron as base metal. Example: steel, alnico etc.
- Non ferrous alloys: They do not contain iron as base metal. Example: brass, bronze, duralumin etc.
The document Materials - Metals & Non-metal Summary Class 8 NCERT Summary is a part of the Grade 8 Course Science for Grade 8.
All you need of Grade 8 at this link: Grade 8
|
61 videos|146 docs|40 tests
|
Related Searches





















