Class 10 Science Chapter 2 Question Answers - Acids, Bases and Salts
Q1: What is dilution? What precaution should be taken during dilution of a strong acid like sulphuric acid?
Ans: Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent.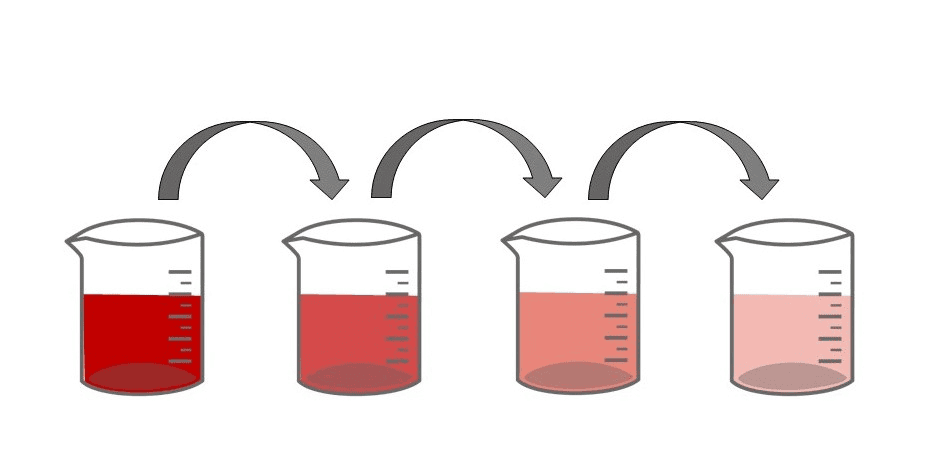 Precaution during Dilution of Strong Acid: When diluting a strong acid like sulphuric acid, always add the acid slowly to water, never the other way around. This prevents the exothermic reaction from causing the acid to splatter, reducing the risk of burns and other accidents.
Precaution during Dilution of Strong Acid: When diluting a strong acid like sulphuric acid, always add the acid slowly to water, never the other way around. This prevents the exothermic reaction from causing the acid to splatter, reducing the risk of burns and other accidents.
Q2: What is tooth enamel chemically? State the conditions when it starts corroding. What happens when food particles left in the mouth after eating degrade? Why do doctors suggest use of powder/tooth paste to prevent tooth decay?
Ans:
- The tooth enamel is chemically calcium phosphate with the formula Ca3(PO4)2. It is quite hard.
- The enamel starts corroding when the pH inside our mouth falls below 5.5 because the saliva present in the mouth becomes acidic.
- The bacteria present in the mouth breakdown the food particles into acids which damage our teeth by corroding them.
- The contents of the tooth paste are of basic nature. They neutralise the excess acid present. As a result, the corrosion of enamel and decay of teeth are checked.
Q3: When zinc metal is treated with a dilute solution of a strong acid, a gas is evolved, which is utilised in the hydrogenation of oil. Name the gas evolved. Write the chemical equation of the reaction involved and also write a test to detect the gas formed.
Ans: Zinc metal gives hydrogen gas when it is treated with dilute sulphuric acid. Hydrogen gas is utilised in hydrogenation of oil.
Therefore, the gas evolved is hydrogen.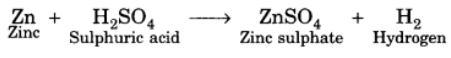 Test for hydrogen gas: When a burning candle is brought near hydrogen gas, it burns with a pop sound which confirms the presence of hydrogen gas.
Test for hydrogen gas: When a burning candle is brought near hydrogen gas, it burns with a pop sound which confirms the presence of hydrogen gas.
Q4: A white powder is added while baking breads and cakes to make them soft and fluffy. Write the name of the powder. Name its main ingredients. Explain the function of each ingredient. Write the chemical reaction taking place when the powder is heated during baking.
Ans: The white powder is known as baking powder. The main ingredients are baking soda (NaHCO3) and tartaric acid (C4H6O6).
Q5: A compound which is prepared from gypsum has the property of hardening when mixed with proper quantity of water. Identify the compound. Write chemical equation to prepare the compound. Mention one important use of the compound.
Ans: The compound is Plaster of Paris 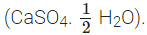 It is formed from gypsum
It is formed from gypsum 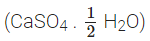 upon heating to a temperature of 373 K. It changes back to gypsum on adding water. Plaster of Paris is used for setting fractured bones.
upon heating to a temperature of 373 K. It changes back to gypsum on adding water. Plaster of Paris is used for setting fractured bones.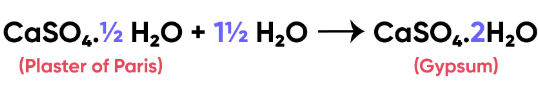
Q6: If 280 g of washing soda crystals are left in dry air for some time, a loss of weight of 162 g occurs. How can you account for this?
Ans: Washing soda (Na2CO3. 10H2O) is an efflorescent substance (if exposed to air, it loses most of its water of crystallisation). 280 g of washing soda lose 162 g of its water of crystallisation.
Q7: A solution of HCl is taken in a beaker and an electric circuit with a bulb is set up with the solution in series. What happens to the bulb and why?
Ans: The bulb will start glowing. Glowing of the bulb indicates that there is a flow of electric current through the solution. Electric current is carried through the solution by ions.
Since the cation present in acids is H+, this suggests that acids produce hydrogen ions, H+(ag), in solution, which are responsible for carrying current through the solution.
Q8: Explain why sodium hydroxide solution cannot be kept in aluminium containers? Write equation for the reaction that may take place for the same.
Ans: Sodium hydroxide solution reacts with aluminium to form sodium metaaluminate and hydrogen is evolved. Therefore, it cannot be kept in a container made of aluminium.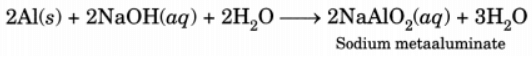
Q9: List two differences between acids and bases on the basis of chemical properties.
Ans:
- Dilute acids like HCl and H2SO4 evolve H2 gas on reacting with metals like Zn, Mg and Ca, etc. and dilute bases do not evolve hydrogen gas.
- Acids react with oxides of metals while bases react with oxides of non-metals.
Q10: Name the acid present in ant sting and give its chemical formula. Also give the common method to get relief from the discomfort caused by the ant sting.
Ans: The acid present in ant sting: Methanoic acid
Chemical Formula of methanoic acid: HCOOH
Method to get relief from the discomfort caused by the ant sting: Rubbing baking soda over the area of ant sting.
Rubbing baking soda (a base) over ant sting neutralises the methanoic acid present in the ant sting and gives relief from pain.
Q11: A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance, which can be moulded into different shapes by making its dough. When this compound is left in the open for some time, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt and why does it show such a behaviour? Give the reaction involved.
Ans: Calcium belongs to group 2. Calcium sulphate is a white soft substance. It is known as Plaster of Paris, which can be moulded into different shapes by making its dough.
When Plaster of Paris is left for some time in the open, it turns into a solid mass because of reaction with moisture present in the atmosphere. The solid mass so formed is known as gypsum and cannot be further used for moulding.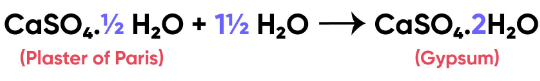 The above said group 2 element is calcium sulpahte.
The above said group 2 element is calcium sulpahte.
Q12: How would you distinguish between baking powder and washing soda by heating?
Ans: Baking soda (NaHCO3) gives carbon dioxide and water vapour on heating at very low temperature. The gas so formed turns lime water milky, which confirms the presence of carbon dioxide gas.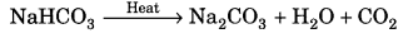 When washing soda (Na2CO3) is heated it does not produce carbon dioxide even at high temperatures, but gives off its water of crystallisation to become anhydrous salt.
When washing soda (Na2CO3) is heated it does not produce carbon dioxide even at high temperatures, but gives off its water of crystallisation to become anhydrous salt.
Q13: Write balanced equations to satisfy each statement:
(a) Acid + Chloride → Salt + Hydrochloric acid gas
(b) Acid + Carbonate → Salt + Water + Carbon dioxide
(c) Acid + Sulphite → Salt + Water + Sulphur dioxide
Ans:
(a) H2SO4 + NaCl → NaHSO4 + HCl (g)
(b) 2HCl + Na2CO3 → 2NaCl + H2O + CO2 (g)
(c) 2HCl + CaSO3 → CaCl2 + H2O + SO2 (g)
Q14: Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain:
(a) Sodium sulphate
(b) Iron (II) sulphate
(c) Zinc carbonate.
Dilute sulphuric acid, copper, iron, copper carbonate, sodium, zinc, sodium carbonate
Ans:
(a) Sodium sulphate
Na2CO3 + H2SO4 (dil.) → Na2SO4 + H2O + CO2 (g)
(b) Iron (II) sulphate
Fe + H2SO4 (dil.) → FeSO4 + H2 (g)
(c) Zinc carbonate
Zn + CuCO3 → ZnCO3 + Cu
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science Chapter 2 Question Answers - Acids, Bases and Salts
| 1. What are some common examples of acids, bases, and salts? |  |
| 2. How do acids and bases differ in terms of their pH levels? |  |
| 3. How do acids, bases, and salts react with each other? |  |
| 4. What are the properties of acids, bases, and salts? |  |
| 5. How are acids, bases, and salts used in everyday life? |  |

















