Class 9 Science Chapter 4 Question Answers - Structure of the Atom
Q1: Number of electrons, protons and neutrons in chemical species A, B, C and D is given below: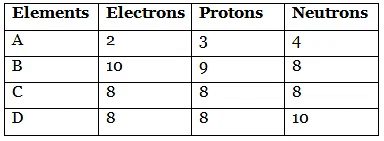
Now, answer the following questions :
(a) What is the mass number of A and B?
(b) What is the atomic number of B?
(c) Which two elements represent a pair of isotopes and why?
(d) What is the valency of element C?
Also, justify your answers.
Ans:
(a) Mass number of A = 3 + 4 = 7
Mass number of B = 9 + 8 = 17
(b) Atomic number of B = Number of protons = 9
(c) Elements C and D represent a pair of isotopes because their atomic numbers are the same.
(d) Electronic configuration of C (8) = 2, 6. So, its valency is 2.
Q2: Describe in brief the Rutherford’s alpha-particle scattering experiment with the help of labelled diagram. Write any three important conclusions drawn from the experiment.
Ans : Rutherford took a very thin gold foil and born bared it with a -particles and he observed that :
(i) Most of the fast moving a-particles passed straight through the gold foil.
(ii) Some of the alpha-particles were deflected by the foil by small angle.
(iii) O ut of every 12000 particles, one appeared to rebound.
From the above observations, he concluded :
(i) There is a positively charged centre in an atom called the nucleus. Nearly all mass of an atom resides in the nucleus.
(ii) The electrons revolve around the nucleus in well defined orbits.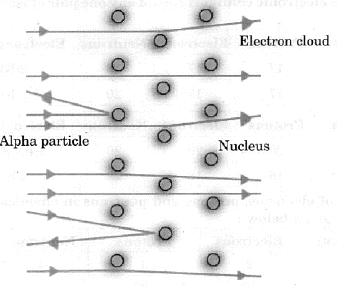 (iii) The size of the nucleus is very small as compared with the size of the atom.
(iii) The size of the nucleus is very small as compared with the size of the atom.
Q3: Explain Rutherford’s atomic model.
Ans: Rutherford purposed a model of an atom on the basis of a-particles scattering experiment. This is known as Rutherford’s nuclear model of atom.
(i) An atom consist, a heavy positively charged core called nucleus.
(ii) Nucleus is surrounded by electrons.
(iii) E lectrons and nucleus are held together by electrostatic force of attraction.
(iv) Size of nucleus is very small as compared to the size of atom.
(v) Almost the entire mass of the atom is concentrated in the nucleus.
Q4: How was the neutron discovered?
Ans: Atom was considered to have electrons and protons only till 1920. But electrons have negligible mass. Then entire mass of the atom was considered to be only due to the protons present in it. In 1920, Rutherford found that atomic masses of all elements are higher than the mass of all protons and electrons in their atoms. Chadwick discovered the presence of an electrically neutral particle inside the atom in 1932.
Q5: Give the number of electron, proton and neutron in 59CO27 and 108Ag47.
Ans :
(i) Number of protons in Co = 27
(ii) Number of electrons in Co = 27
(iii) Number of neutrons in Co = 59 – 27 = 32
(iv) Number of protons in Ag = 47
(v) Number of electrons in Ag = 47
(vi) Number of neutrons in Ag = 108 – 47 = 61
Q6: Give difference between isotopes and isobars.
Ans: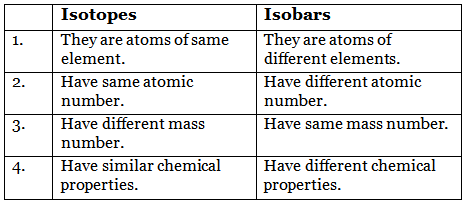
Q7: Chlorine occurs in nature in two isotopic forms with masses 35u and 37u in the ratio of 3 : 1. What should be the mass of chlorine atom?
Ans: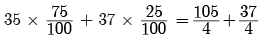
= 142/4
= 35.5u
Q8: An element 12X24 loses two electrons to form a cation which combines with the anion of element 17Y35 formed by gaining an electron.
(i) Write the electronic configuration of element X.
(ii) Write the electronic configuration of the anion of element Y.
(iii) Write the formula for the compound formed by combination of X and Y.
Ans:
(i) X = 2, 8, 2
(ii) Y = 2, 8, 8
(iii) XY2
Q9: Elaborate the postulates put forward by E. Rutherford about the structure of atom based on the a -particle scattering experiment.
Ans :
(i) Most of the space inside the atom is empty because most of the a -particles passed through the gold foil without getting deflected.
(ii) Very few particles are deflected from their path, indicating that positive charge of the atom occupies very little space.
(iii) A very small fraction of particles was deflected by 180°, indicating that all the positive charge and mass of the gold atom were concentrated in a small volume within the atom.
Q10: Give reasons :
(i) Mass number of an atom excludes the mass of an electron.
(ii) Nucleus of an atom is charged.
(iii) Alpha-particle scattering experiment was possible by using gold foil only and not by foil of any other metal.
Ans:
(i) Mass number of an atom excludes the mass of an electron because electrons have negligible mass in comparison to protons and neutrons.
(ii) Nucleus of an atom consists of protons and neutrons. Protons are positively charged particles. So, the nucleus of an atom is charged.
(iii) Because an extremely thin film was required for the experiment and it was only possible by using gold, as gold is a highly malleable metal.
Q11: Give the postulates of Dalton’s atomic theory.
Ans:
(i) Every element is composed of extremely small particles called atoms.
(ii) Atoms of a given element are identical, both in mass and properties. Different chemical elements have different kinds of atoms; in particular, their atoms have different masses.
(iii) Atoms cannot be created, destroyed or transformed into atoms of other elements.
(iv) Compounds are formed when atoms of different elements combine with each other in small whole number ratios.
(v) The relative number and kinds of atoms in a given compound are constant.
|
84 videos|384 docs|61 tests
|






















