Important Diagrams: Matter in Our Surroundings | Science Class 9 PDF Download
Q1: Answer the following questions based on the diagram given below:
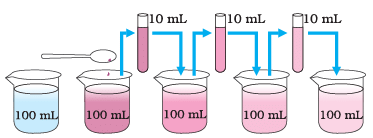
(i) What is the purpose of the experiment mentioned in the diagram?
Ans: The purpose of the experiment is to estimate the size of particles in matter by diluting a substance and observing how the color changes with each dilution.
(ii) How does the color of the substance change as it is diluted?
Ans: As the substance is diluted, its color becomes lighter, but it remains visible.
(iii) What does the visibility of the color in the experiment suggest about the particles in matter?
Ans: The visibility of the color even after dilution suggests that the particles in matter are very small and not visible to the naked eye.
(iv) Why is it important to dilute the substance in this experiment?
Ans: Diluting the substance allows us to observe how the color changes gradually, which helps in estimating the size of particles in matter more accurately.
(v) How does this experiment relate to the concept of the particulate nature of matter?
Ans: This experiment demonstrates the particulate nature of matter by showing that matter is composed of tiny particles (atoms or molecules) that are so small they can remain suspended in a liquid even after multiple dilutions, making them visible as a color.
Q2: Answer the following questions based on the diagram given below: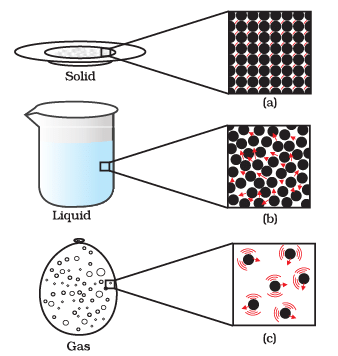
(i) Describe the motion of particles in a solid state of matter.
Ans: In a solid state of matter, particles vibrate in fixed positions and do not move from one place to another. They have the least amount of freedom in terms of motion.
(ii) How does the motion of particles differ in a liquid compared to a solid?
Ans: In a liquid state of matter, particles are still closely packed but have more freedom to move around. They can slide past each other, allowing liquids to flow and take the shape of the container.
(iii) Explain the motion of particles in a gaseous state.
Ans: In a gaseous state of matter, particles have a lot of freedom and move randomly at high speeds. They fill the entire available space and do not have a fixed shape or volume.
(iv) Which state of matter has the most organized particle motion and why?
Ans: A solid state of matter has the most organized particle motion because the particles vibrate in fixed positions and maintain a specific arrangement, giving solids a definite shape and volume.
(v) How does heating a solid or a liquid affect the motion of its particles?
Ans: Heating a solid or a liquid increases the motion of its particles. In solids, heating makes particles vibrate more vigorously. In liquids, it allows particles to move faster and further apart, changing the state from solid to liquid or liquid to gas, depending on the temperature increase.
Q3: Answer the following questions based on the diagram given below: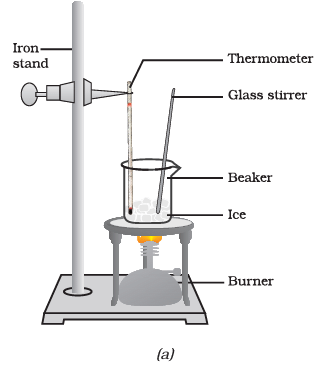
(i) What is the purpose of suspending the laboratory thermometer in the beaker with the ice?
Ans: The purpose of suspending the laboratory thermometer in the beaker with the ice is to measure and monitor the temperature changes during the process of ice melting and water boiling.
(ii) At what temperature does the ice start melting in this experiment?
Ans: The temperature at which the ice starts melting is recorded when it reaches its melting point, which is 0 degrees Celsius.
(iii) What do you observe when all the ice in the beaker has converted into water?
Ans: When all the ice in the beaker has converted into water, you observe that the temperature remains constant at 0 degrees Celsius until all the ice has melted. This is known as the plateau or plateau phase.
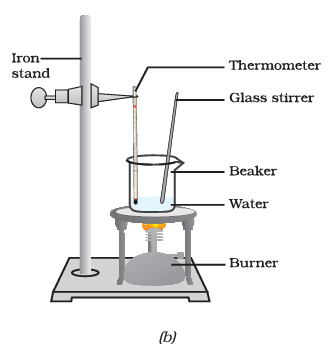
(iv) What happens to the temperature of the water in the beaker when it starts boiling after the glass rod is heated and stirred?
Ans: When the water in the beaker starts boiling after heating and stirring with the glass rod, the temperature remains constant at 100 degrees Celsius until most of the water has vaporized. This is the boiling point of water, and the temperature does not rise further during this phase.
(v) What are the two conversions of states observed in this experiment, and what are their corresponding temperature plateaus?
Ans: In this experiment, two state conversions are observed:
- Solid to Liquid: The conversion of ice to water. The temperature remains constant at 0 degrees Celsius during this phase.
- Liquid to Gaseous: The conversion of liquid water to water vapor (steam). The temperature remains constant at 100 degrees Celsius during this phase.
Q4: Answer the following questions based on the diagram given below: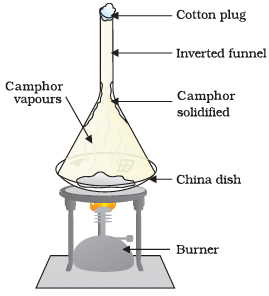
(i) Describe the experiment shown in Figure.
Ans: In the experiment shown in Figure, we take some camphor, crush it, and place it in a china dish. Then, we place an inverted funnel over the china dish with a cotton plug on the stem of the funnel. Finally, we heat the setup slowly and observe the changes.
(ii) What is the purpose of using the inverted funnel and cotton plug in this experiment?
Ans: The inverted funnel with a cotton plug is used to collect the vapors produced during the heating process and prevent them from escaping. It helps in observing and studying the substances released during the experiment.
(iii) What happens to the camphor when it is heated in this experiment?
Ans: When camphor is heated in this experiment, it undergoes sublimation. It directly changes from a solid state to a gaseous state without passing through the liquid state.
(iv) What can you infer from the observation of this experiment?
Ans: From this experiment, we can infer that camphor undergoes sublimation when heated. It transforms from a solid to a gas without melting into a liquid. This demonstrates the sublimation property of camphor.
(v) Why is it important to use a china dish in this experiment?
Ans: A china dish is used in this experiment because it can withstand high temperatures without breaking. Since we are heating the camphor, it is essential to use a heat-resistant dish to avoid any damage or accidents.
Q5: Answer the following questions based on the diagram given below: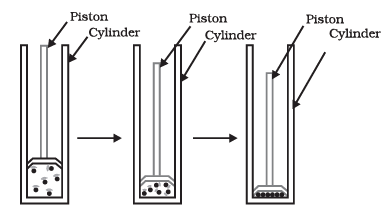
(i) What happens to a gas when it is compressed under pressure in a cylinder?
Ans: When a gas is compressed under pressure in a cylinder, its constituent particles come closer together.
(ii) How does pressure affect the state of matter?
Ans: Increasing pressure can change a gas into a liquid. Reducing pressure can turn a gas back into a gaseous state.
(iii) What is the result of applying pressure and reducing temperature to gases?
Ans: Applying pressure and reducing temperature can liquefy gases, transforming them into a liquid state.
(iv) What is solid carbon dioxide (CO2) commonly known as, and how is it stored?
Ans: Solid carbon dioxide is known as dry ice and is stored under high pressure.
(v) Describe the transformation of solid carbon dioxide (CO2) when pressure decreases to 1 atmosphere.
Ans: Solid CO2 directly converts into a gaseous state when the pressure decreases to 1 atmosphere without transitioning into a liquid state. This transformation is why solid carbon dioxide is called dry ice.
Q6: Answer the following questions based on the diagram given below: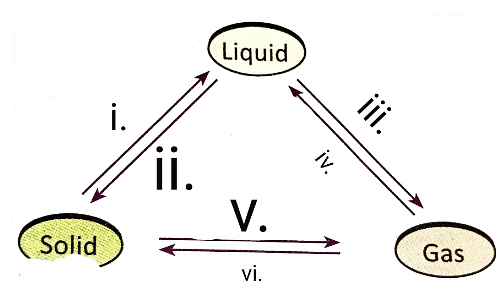
(i) How does applying pressure and reducing temperature affect gases?
Ans: Applying pressure and reducing temperature can liquefy gases.
(ii) What happens when solid carbon dioxide (CO2) is exposed to 1 atmosphere of pressure?
Ans: Solid CO2 (dry ice) directly converts into the gaseous state without becoming liquid.
(iii) How does pressure influence the state of matter according to the information provided?
Ans: Pressure, along with temperature, determines whether a substance will be in a solid, liquid, or gas state.
(iv) What is the reason solid carbon dioxide is called "dry ice"?
Ans: Solid carbon dioxide is called "dry ice" because it directly turns into a gas without becoming a liquid when pressure is decreased to 1 atmosphere.
(v) In the context of the experiment on interconversion of matter, what two factors determine the state of a substance?
Ans: Temperature and pressure are the two factors that determine the state of a substance, whether it will be solid, liquid, or gas.
|
84 videos|384 docs|61 tests
|
FAQs on Important Diagrams: Matter in Our Surroundings - Science Class 9
| 1. What are the different states of matter? |  |
| 2. How does matter change from one state to another? |  |
| 3. What is the significance of the diagram for understanding matter in our surroundings? |  |
| 4. How does temperature affect the state of matter? |  |
| 5. What are the characteristics of each state of matter? |  |






















