Atomic Orbital Shapes: Radial and Angular Wave Functions | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Introduction |

|
| The Angular Component |

|
| Electron Spin: The Fourth Quantum Number |

|
| Solved Examples |

|
Introduction
- The first six radial functions are provided in Table 4.10.2. Note that the functions in the table exhibit a dependence on Z, the atomic number of the nucleus. Other one-electron systems have electronic states analogous to those for the hydrogen atom, and inclusion of the charge on the nucleus allows the same wavefunctions to be used for all one-electron systems. For hydrogen, Z = 1 and for helium, Z = 2.
Table 4.10.2: Hydrogen-like atomic wavefunctions for n values 1, 2, 3: Z is the atomic number of the nucleus, and ρ = Zr/a0, where α0 is the Bohr radius and r is the radial variable.

- Visualizing the variation of an electronic wavefunction with r, θ, and ϕ is important because the absolute square of the wavefunction depicts the charge distribution (electron probability density) in an atom or molecule. The charge distribution is central to chemistry because it is related to chemical reactivity. For example, an electron deficient part of one molecule is attracted to an electron rich region of another molecule, and such interactions play a major role in chemical interactions ranging from substitution and addition reactions to protein folding and the interaction of substrates with enzymes.
- Methods for separately examining the radial portions of atomic orbitals provide useful information about the distribution of charge density within the orbitals. Graphs of the radial functions, R(r), for the 1s, 2s, and 2p orbitals plotted in Figure 4.10.2 left). The quantity R(r)∗R(r) gives the radial probability density; i.e., the probability density for the electron to be at a point located the distance r from the proton. Radial probability densities for three types of atomic orbitals are plotted in Figure 4.10.2 (right).


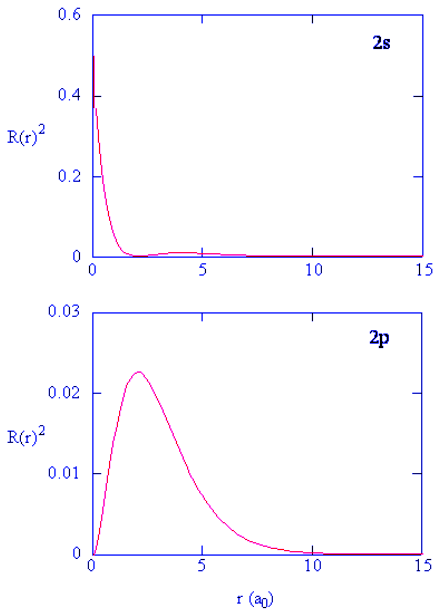 Figure 4.10.2: (left) Radial function, R(r), for the 1s, 2s, and 2p orbitals. (right) Radial probability densities for the 1s, 2s, and 2p orbitals.
Figure 4.10.2: (left) Radial function, R(r), for the 1s, 2s, and 2p orbitals. (right) Radial probability densities for the 1s, 2s, and 2p orbitals.
- For the hydrogen atom, the peak in the radial probability plot occurs at r = 0.529 Å (52.9 pm), which is exactly the radius calculated by Bohr for the n = 1 orbit. Thus the most probable radius obtained from quantum mechanics is identical to the radius calculated by classical mechanics. In Bohr’s model, however, the electron was assumed to be at this distance 100% of the time, whereas in the Schrödinger model, it is at this distance only some of the time. The difference between the two models is attributable to the wavelike behavior of the electron and the Heisenberg uncertainty principle.
- Figure 4.10.3 compares the electron probability densities for the hydrogen 1s, 2s, and 3s orbitals. Note that all three are spherically symmetrical. For the 2s and 3s orbitals, however (and for all other s orbitals as well), the electron probability density does not fall off smoothly with increasing r. Instead, a series of minima and maxima are observed in the radial probability plots (part (c) in Figure 4.10.3). The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero electron probability.


Figure 4.10.3: Probability Densities for the 1 s, 2 s, and 3s Orbitals of the Hydrogen Atom. (a) The electron probability density in any plane that contains the nucleus is shown. Note the presence of circular regions, or nodes, where the probability density is zero. (b) Contour surfaces enclose 90% of the electron probability, which illustrates the different sizes of the 1s, 2s, and 3s orbitals. The cutaway drawings give partial views of the internal spherical nodes. The orange color corresponds to regions of space where the phase of the wave function is positive, and the blue color corresponds to regions of space where the phase of the wave function is negative. (c) In these plots of electron probability as a function of distance from the nucleus (r) in all directions (radial probability), the most probable radius increases asn increases, but the 2s and 3s orbitals have regions of significant electron probability at small values of r.
The Angular Component
- The angular component of the wavefunction Y(θ,ϕ) in Equation 4.10.4 does much to give an orbital its distinctive shape. Y(θ, ϕ) is typically normalized so the the integral of Y2(θ, ϕ)over the unit sphere is equal to one. In this case, Y2(θ, ϕ) serves as a probability function. The probability function can be interpreted as the probability that the electron will be found on the ray emitting from the origin that is at angles (θ, ϕ) from the axes.
- The probability function can also be interpreted as the probability distribution of the electron being at position (θ, ϕ) on a sphere of radius r, given that it is r distance from the nucleus. Yl, ml(θ, ϕ) are also the wavefunction solutions to Schrödinger’s equation for a rigid rotor consisting of rotating bodies, for example a diatomic molecule. These are called Spherical Harmonic functions (Table M4).
s Orbitals (l=0)
Three things happen to s orbitals as n increases (Figure 6.6.2):
- They become larger, extending farther from the nucleus.
- They contain more nodes. This is similar to a standing wave that has regions of significant amplitude separated by nodes, points with zero amplitude.
- For a given atom, the s orbitals also become higher in energy as n increases because of their increased distance from the nucleus.
Orbitals are generally drawn as three-dimensional surfaces that enclose 90% of the electron density. Although such drawings show the relative sizes of the orbitals, they do not normally show the spherical nodes in the 2s and 3s orbitals because the spherical nodes lie inside the 90% surface. Fortunately, the positions of the spherical nodes are not important for chemical bonding.
p Orbitals (l=1)
- Only s orbitals are spherically symmetrical. As the value of l increases, the number of orbitals in a given subshell increases, and the shapes of the orbitals become more complex. Because the 2p subshell has l = 1, with three values of ml (−1, 0, and +1), there are three 2p orbitals).

Figure 6.6.2, the colors correspond to regions of space where the phase of the wave function is positive (orange) and negative (blue).
- The electron probability distribution for one of the hydrogen 2p orbitals is shown in Figure 4.10.4 . Because this orbital has two lobes of electron density arranged along the z axis, with an electron density of zero in the xy plane (i.e., the xy plane is a nodal plane), it is a 2pz orbital. As shown in Figure 4.10.5, the other two 2p orbitals have identical shapes, but they lie along the x axis (2px) and y-axis (2py), respectively. Note that each p orbital has just one nodal plane. In each case, the phase of the wave function for each of the 2p orbitals is positive for the lobe that points along the positive axis and negative for the lobe that points along the negative axis. It is important to emphasize that these signs correspond to the phase of the wave that describes the electron motion, not to positive or negative charges.

- The surfaces shown enclose 90% of the total electron probability for the 2px, 2py, and 2pz orbitals. Each orbital is oriented along the axis indicated by the subscript and a nodal plane that is perpendicular to that axis bisects each 2p orbital. The phase of the wave function is positive (orange) in the region of space where x, y, or z is positive and negative (blue) where x, y, or z is negative. Just as with the s orbitals, the size and complexity of the p orbitals for any atom increase as the principal quantum number n increases. The shapes of the 90% probability surfaces of the 3p, 4p, and higher-energy p orbitals are, however, essentially the same as those shown in Figure Figure 4.10.5
d Orbitals (l=2)
Subshells with l = 2 have five d orbitals; the first principal shell to have a d subshell corresponds to n = 3. The five d orbitals have ml values of −2, −1, 0, +1, and +2 (Figure 4.10.6).
Figure 4.10.6: The Five Equivalent 3d Orbitals of the Hydrogen Atom. The surfaces shown enclose 90% of the total electron probability for the five hydrogen 3d orbitals. Four of the five 3d orbitals consist of four lobes arranged in a plane that is intersected by two perpendicular nodal planes. These four orbitals have the same shape but different orientations. The fifth 3d orbital, 3dz2, has a distinct shape even though it is mathematically equivalent to the others. The phase of the wave function for the different lobes is indicated by color: orange for positive and blue for negative.
- The hydrogen 3d orbitals have more complex shapes than the 2p orbitals. All five 3d orbitals contain two nodal surfaces, as compared to one for each p orbital and zero for each s orbital. In three of the d orbitals, the lobes of electron density are oriented between the x and y, x and z, and y and z planes; these orbitals are referred to as the 3dxy, \)3d_{xz}\_, and \)3d_{yz}\) orbitals, respectively. A fourth d orbital has lobes lying along the x and y axes; this is the 3dx2−y2 orbital. The fifth 3d orbital, called the 3dz2 orbital, has a unique shape: it looks like a 2pz orbital combined with an additional doughnut of electron probability lying in the xy plane.
- Despite its peculiar shape, the 3dz2 orbital is mathematically equivalent to the other four and has the same energy. In contrast to p orbitals, the phase of the wave function for d orbitals is the same for opposite pairs of lobes. As shown in Figure 4.10.6, the phase of the wave function is positive for the two lobes of the dz2 orbital that lie along the z axis, whereas the phase of the wave function is negative for the doughnut of electron density in the xy plane. Like the s and p orbitals, as n increases, the size of the d orbitals increases, but the overall shapes remain similar to those depicted in Figure 6.6.5.
f Orbitals (l = 3)
Principal shells with n = 4 can have subshells with l = 3 and ml values of −3, −2, −1, 0, +1, +2, and +3. These subshells consist of seven f orbitals. Each f orbital has three nodal surfaces, so their shapes are complex (not shown).
Energies
- The constraint that n be greater than or equal to l + 1 also turns out to quantize the energy, producing the same quantized expression for hydrogen atom energy levels that was obtained from the Bohr model of the hydrogen atom.
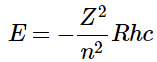 (4.10.9)
(4.10.9)
or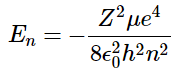 (4.10.10)
(4.10.10) - The relative energies of the atomic orbitals with n ≤ 4 for a hydrogen atom are plotted in Figure 4.10.7; note that the orbital energies depend on only the principal quantum number n. Consequently, the energies of the 2s and 2p orbitals of hydrogen are the same; the energies of the 3s, 3p, and 3d orbitals are the same; and so forth. The orbital energies obtained for hydrogen using quantum mechanics are exactly the same as the allowed energies calculated by Bohr.
- In contrast to Bohr’s model, however, which allowed only one orbit for each energy level, quantum mechanics predicts that there are 4 orbitals with different electron density distributions in the n = 2 principal shell (one 2s and three 2p orbitals), 9 in the n = 3 principal shell, and 16 in the n = 4 principal shell.
Note:
For a single electron system, the energy of that electron is a function of only the principal quantum number (Equation 4.10.9 ).
- The different values of l and ml for the individual orbitals within a given principal shell are not important for understanding the emission or absorption spectra of the hydrogen atom under most conditions, but they do explain the splittings of the main lines that are observed when hydrogen atoms are placed in a magnetic field. As we have just seen, however, quantum mechanics also predicts that in the hydrogen atom, all orbitals with the same value of n (e.g., the three 2p orbitals) are degenerate, meaning that they have the same energy. Figure 4.10.7 shows that the energy levels become closer and closer together as the value of n increases, as expected because of the 1/n2 dependence of orbital energies.

Figure 4.10.7: Orbital Energy Level Diagram for the Hydrogen Atom. Each box corresponds to one orbital. Note that the difference in energy between orbitals decreases rapidly with increasing values of n.
- In general, both energy and radius decrease as the nuclear charge increases. Thus the most stable orbitals (those with the lowest energy) are those closest to the nucleus. For example, in the ground state of the hydrogen atom, the single electron is in the 1s orbital, whereas in the first excited state, the atom has absorbed energy and the electron has been promoted to one of the n = 2 orbitals. In ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as the px, py, and pz orbitals, are degenerate.
Note: Bohr vs. Schrödinger
It is interesting to compare the results obtained by solving the Schrödinger equation with Bohr’s model of the hydrogen atom. There are several ways in which the Schrödinger model and Bohr model differ.
- First, and perhaps most strikingly, the Schrödinger model does not produce well-defined orbits for the electron. The wavefunctions only give us the probability for the electron to be at various directions and distances from the proton.
- Second, the quantization of angular momentum is different from that proposed by Bohr. Bohr proposed that the angular momentum is quantized in integer units of ℏ, while the Schrödinger model leads to an angular momentum of (l(l + 1)ℏ2)1/2 .
- Third, the quantum numbers appear naturally during solution of the Schrödinger equation while Bohr had to postulate the existence of quantized energy states. Although more complex, the Schrödinger model leads to a better correspondence between theory and experiment over a range of applications that was not possible for the Bohr model.
Electron Spin: The Fourth Quantum Number
- The quantum numbers n, l, m are not sufficient to fully characterize the physical state of the electrons in an atom. In 1926, Otto Stern and Walther Gerlach carried out an experiment that could not be explained in terms of the three quantum numbers n, l, m and showed that there is, in fact, another quantum-mechanical degree of freedom that needs to be included in the theory. The experiment is illustrated in the Figure 4.10.8.
- A beam of atoms (e.g. hydrogen or silver atoms) is sent through a spatially inhomogeneous magnetic field with a definite field gradient toward one of the poles. It is observed that the beam splits into two beams as it passes through the field region.

Figure 4.10.8: The Stern-Gerlach apparatus.
- The fact that the beam splits into 2 beams suggests that the electrons in the atoms have a degree of freedom capable of coupling to the magnetic field. That is, an electron has an intrinsic magnetic moment M arising from a degree of freedom that has no classical analog. The magnetic moment must take on only 2 values according to the Stern-Gerlach experiment.
- The intrinsic property that gives rise to the magnetic moment must have some analog to a spin, S; unlike position and momentum, which have clear classical analogs, spin does not. The Stern-Gerlach experiment implies that we need to include a fourth quantum number, ms in our description of the physical state of the electron. That is, in addition to give its principle, angular, and magnetic quantum numbers, we also need to say if it is a spin-up electron or a spin-down electron.
Note: Spin is a Quantum Phenomenon
- Unlike position and momentum, which have clear classical analogs, spin does not.
George Uhlenbeck (1900–1988) and Samuel Goudsmit (1902–1978), proposed that the splittings were caused by an electron spinning about its axis, much as Earth spins about its axis. When an electrically charged object spins, it produces a magnetic moment parallel to the axis of rotation, making it behave like a magnet. Although the electron cannot be viewed solely as a particle, spinning or otherwise, it is indisputable that it does have a magnetic moment. This magnetic moment is called electron spin.
Figure 4.10.9: Electron Spin. In a magnetic field, an electron has two possible orientations with different energies, one with spin up, aligned with the magnetic field, and one with spin down, aligned against it. All other orientations are forbidden.
- In an external magnetic field, the electron has two possible orientations (Figure 4.10.9). These are described by a fourth quantum number (ms), which for any electron can have only two possible values, designated +½ (up) and −½ (down) to indicate that the two orientations are opposites; the subscript s is for spin. An electron behaves like a magnet that has one of two possible orientations, aligned either with the magnetic field or against it.
- The implications of electron spin for chemistry were recognized almost immediately by an Austrian physicist, Wolfgang Pauli (1900–1958; Nobel Prize in Physics, 1945), who determined that each orbital can contain no more than two electrons whoe developed the Pauli exclusion principle.
Note: Pauli Exclusion Principle
- No two electrons in an atom can have the same values of all four quantum numbers (n, l, ml, ms).
By giving the values of n, l, and ml, we also specify a particular orbital (e.g., 1s with n = 1, l = 0, ml = 0). Because ms has only two possible values (+½ or −½), two electrons, and only two electrons, can occupy any given orbital, one with spin up and one with spin down. With this information, we can proceed to construct the entire periodic table, which was originally based on the physical and chemical properties of the known elements.
Solved Examples
Example 1: List all the allowed combinations of the four quantum numbers (n, l, ml, ms) for electrons in a 2p orbital and predict the maximum number of electrons the 2p subshell can accommodate.
Given: orbital
Asked for: allowed quantum numbers and maximum number of electrons in orbital
Strategy:
List the quantum numbers (n, l, ml) that correspond to an n = 2p orbital. List all allowed combinations of (n, l, ml).
Build on these combinations to list all the allowed combinations of (n, l, ml, ms).
Add together the number of combinations to predict the maximum number of electrons the 2p subshell can accommodate.
Ans: (a) For a 2p orbital, we know that n = 2, l = n − 1 = 1, and ml = −l, (−l +1),…, (l − 1), l. There are only three possible combinations of (n, l, ml): (2, 1, 1), (2, 1, 0), and (2, 1, −1).
(b) Because ms is independent of the other quantum numbers and can have values of only +½ and −½, there are six possible combinations of (n, l, ml, ms): (2, 1, 1, +½), (2, 1, 1, −½), (2, 1, 0, +½), (2, 1, 0, −½), (2, 1, −1, +½), and (2, 1, −1, −½).
(c) Hence the 2p subshell, which consists of three 2p orbitals (2px, 2py, and 2pz), can contain a total of six electrons, two in each orbital.
Example 2: List all the allowed combinations of the four quantum numbers (n, l, ml, ms) for a 6s orbital, and predict the total number of electrons it can contain.
Ans: (6, 0, 0, +½), (6, 0, 0, −½); two electrons
FAQs on Atomic Orbital Shapes: Radial and Angular Wave Functions - Chemistry Optional Notes for UPSC
| 1. What is an Angular Component? |  |
| 2. How does Electron Spin relate to the Fourth Quantum Number? |  |
| 3. What are Atomic Orbital Shapes? |  |
| 4. What are Radial and Angular Wave Functions? |  |
| 5. How can I prepare for UPSC exams related to the given article's content? |  |



















