UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Comparison of Valence Bond and Molecular Orbital Theories
Comparison of Valence Bond and Molecular Orbital Theories | Chemistry Optional Notes for UPSC PDF Download
Introduction
- In Quantum chemistry, valence bond (VB) theory and molecular orbital (MO) theory, are two basic theories that are used to explain chemical bonding or simply how atoms come together to form molecules.
- Valence Bond Theory, rooted in the early 20th century, paints a picture of chemical bonds as localized interactions between overlapping atomic orbitals. VBT emphasizes the role of electrons in atomic orbitals that contribute to the formation of covalent bonds. It relies on the concept of resonance to explain molecules with multiple bond configurations, providing a tangible understanding of molecular shapes and hybridization.
- Molecular Orbital Theory on the other hand, considers molecules as entities governed by the interaction of molecular orbitals that span the entire molecule, rather than being confined to individual atoms. It introduces the concept of bonding and antibonding orbitals, offering an elegant explanation for a wide range of molecular properties such as bond strengths, electronic spectra and magnetic behaviors.
Valence bond theory (VBT)
- Valence Bond theory emerged during the early 20th century as part of the development of quantum mechanics and its application to understanding chemical phenomena. It was first proposed by W. Heitler and F. London in 1927. It was formulated as an alternative to the older Lewis electron-dot structures that described bonding in terms of shared electron pairs.
- According to the valence bond theory, “Electrons in a molecule occupy atomic orbitals rather than molecular orbitals. The atomic orbitals overlap on the bond formation and the larger the overlap the stronger the bond.”
- This Theory was developed in order to explain chemical bonding using the method of quantum mechanics. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds when a molecule is formed. This theory primarily focuses on the formation of individual bonds from the atomic orbitals of the participating atoms during the formation of a molecule. In contrast, the molecular orbital theory has orbitals that cover the whole molecule.
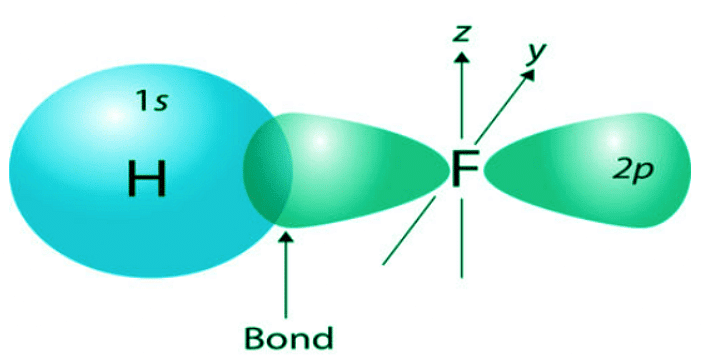
- The central concept of VB theory is that chemical bonds are formed by the overlapping of atomic orbitals from adjacent atoms. The electrons in these overlapping orbitals are shared between the atoms, resulting in a covalent bond.
- Valence bond theory assumes that electrons in a molecule are simply the electrons in the original atomic orbitals, with some used while bonding. In other words, VBT is based on localized bond approach, in which it assumes that the electrons in a molecule occupy atomic orbitals for the individual atoms.
- The strength of the covalent bond is proportional to the degree of overlap between the participating atomic orbitals. When two atoms approach each other, their atomic orbitals may overlap in various ways, leading to different types of covalent bonds, such as sigma (σ) bonds and pi (π) bonds. In other words, Sigma and pi bonds are part of valence bond theory.
- Sigma (σ) Bonds: Formed by the head-on overlap of two atomic orbitals, typically s orbitals or the end-on overlap of p orbitals.
- Pi (π) Bonds: Formed by the lateral overlap of two p orbitals that are parallel to each other. Pi bonds are weaker than sigma bonds.
- VB theory introduces the concept of hybridization, which explains the observed molecular geometries. When atoms form bonds, their atomic orbitals can mix or hybridize to create new hybrid orbitals that are used for bond formation. Hybridization is used to explain molecular shapes and bond angles that cannot be explained using pure atomic orbitals.
- VB theory is capable of explaining multiple bonding (double and triple bonds) by considering the overlap of different sets of orbitals. In cases where multiple Lewis structures are possible, VB theory can account for this through the concept of resonance, where multiple structures contribute to the actual molecular structure.
Question for Comparison of Valence Bond and Molecular Orbital Theories
Try yourself:
Which theory considers molecules as entities governed by the interaction of molecular orbitals that span the entire molecule?View Solution
Applications of Valence Bond Theory
- It is particularly useful for understanding the phenomenon of resonance in molecules. It explains how multiple Lewis structures contribute to the actual structure of a molecule through the overlap of different atomic orbitals.
- It serves as a foundation for the Ligand Field theory, which explains the colors and spectroscopic properties of coordination compounds. It considers the interactions between metal d orbitals and surrounding ligands.
- It is a key concept in explaining the phenomenon of hybridization, where atomic orbitals mix to form hybrid orbitals. This is essential for understanding molecular shapes and the arrangement of atoms around a central atom.
- It can provide insights into the concept of aromaticity, especially in simple cases like benzene. It explains the stability of aromatic compounds by considering the delocalization of electrons in a ring of overlapping p orbitals.
Limitations of Valence Bond Theory
- It struggles to adequately describe molecules with extensive electron delocalization, such as aromatic compounds like benzene.
- For large and complex molecules, the theory becomes increasingly impractical due to the complexity of considering all the potential interactions between atomic orbitals.
- It doesn’t inherently address the Pauli exclusion principle or the antisymmetry requirement of the wave function for indistinguishable electrons.
- It lacks the quantitative predictive power of more advanced theories like MO theory or density functional theory (DFT). It’s often difficult to accurately predict quantitative values using VB theory alone.
- Primarily focuses on explaining bonding and geometry but doesn’t readily extend to explaining properties like molecular orbital energies, ionization potentials, or electron affinities.
Molecular Orbital Theory (MOT)
- Molecular orbital (MO) theorydescribes the behavior of electrons in a molecule in terms of combinations of the atomic wavefunctions. In other words, it is a concept that describes the behavior of electrons in molecules using the principles of quantum mechanics. MOT was first proposed by F. Hund and R.S. Mulliken in 1932.
- It’s an extension of the atomic orbital theory, which describes the behavior of electrons in isolated atoms. MO theory provides a more accurate understanding of chemical bonding, molecular structure, and various properties of molecules.
- When simple bonding occurs between two atoms, the pair of electrons forming the bond occupies a molecular orbital that is a mathematical combination of the wave functions of the atomic orbitals of the two atoms involved.
- Molecular Orbital theory is based on the concept of combining atomic orbitals of individual atoms to form molecular orbitals of the molecule. This is known as the Linear Combination of Atomic Orbitals (LCAO) approach. The basic idea is that atomic orbitals overlap and interact when atoms come together to form a molecule.

- Atomic orbitals are mathematical functions that describe the probability distribution of finding an electron in a particular region around an atomic nucleus. These orbitals are characterized by their principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (m_l), and spin quantum number (m_s).
- When atomic orbitals combine, they create molecular orbitals. Molecular orbitals are different from atomic orbitals in that they span the entire molecule rather than being localized on a specific atom. These molecular orbitals can be bonding, antibonding, or nonbonding, depending on their energy levels and the phase relationship between the combining atomic orbitals.
- Molecular orbitals that result from the constructive interference of atomic orbitals with the same phase are called bonding orbitals. They have lower energy than the original atomic orbitals and contribute to the stability of the molecule. Conversely, molecular orbitals that result from the destructive interference of atomic orbitals with opposite phases are called antibonding orbitals. They have higher energy and contribute to destabilizing the molecule.
- Molecular orbitals are filled with electrons according to the Aufbau principle, Hund’s rule, and the Pauli exclusion principle, which are the same principles that govern the filling of atomic orbitals. Electrons fill the molecular orbitals from the lowest energy level upwards, following the order of increasing energy.
- Molecular Orbital theory provides insights into various molecular properties, such as bond length, bond energy, molecular stability, and electronic properties. It also explains phenomena like paramagnetism and diamagnetism.
- Molecular Orbital theory has its limitations, particularly for larger molecules. Calculations become computationally intensive, and approximations are often made to simplify the calculations. One common approximation is the Hartree-Fock method.
Question for Comparison of Valence Bond and Molecular Orbital Theories
Try yourself:
What is the main application of Molecular Orbital (MO) theory in solid-state physics?View Solution
Applications of Molecular Bond Theory
- MO theory explains the formation of covalent, ionic, and metallic bonds and helps predict molecular geometries, bond lengths, and bond angles. It also clarifies the concept of resonance in molecules.
- It allows researchers to predict the relative strengths of chemical bonds within molecules. Stronger bonds correspond to lower-energy bonding molecular orbitals, while weaker bonds involve higher-energy antibonding molecular orbitals.
- It is used to interpret various spectroscopic techniques, including UV-visible spectroscopy, infrared spectroscopy, and NMR spectroscopy. It helps explain the energy levels and transitions of electrons within molecules.
- In solid-state physics, MO theory is applied to understand the electronic structure and properties of semiconductors. It helps explain concepts such as energy bands, band gaps, and electron and hole behavior in semiconductor materials.
- It can be used to predict various molecular properties, such as ionization potential, electron affinity, and dipole moments.
- In biochemistry and medicinal chemistry, it is employed to understand the interaction of drugs with biological molecules. It helps predict the binding affinities of molecules to protein targets and assists in rational drug design.
 |
Download the notes
Comparison of Valence Bond and Molecular Orbital Theories
|
Download as PDF |
Download as PDF
Limitations of Molecular Orbital Theory
- It involves complex mathematical calculations, especially for large molecules. Solving the Schrödinger equation to determine molecular orbital energies and electron distributions becomes computationally demanding and time-consuming for systems with a high number of atoms or electrons.
- To make calculations more tractable, various approximations are often used in MO theory. For instance, the Hartree-Fock approximation assumes that electron-electron interactions are averaged, neglecting the dynamic correlation between electrons. While these approximations speed up calculations, they can lead to inaccuracies in predicting molecular properties.
- In some cases, the theory can yield results that don’t match experimental observations. For example, MO theory can predict that certain molecules with unpaired electrons are stable and have low energy, even though such species are often highly reactive or short-lived in reality.
- MO theory struggles to accurately describe systems with strong electron-electron correlations, such as transition metal complexes and systems with open-shell electronic configurations.
- The theory, particularly in its mathematical form, might not always provide intuitive chemical explanations for the behavior of complex molecules. It can be challenging to interpret intricate molecular orbital diagrams or understand the physical implications of complex interactions in large systems.
Question for Comparison of Valence Bond and Molecular Orbital Theories
Try yourself:
What is the main difference between Valence Bond Theory and Molecular Orbital Theory?View Solution
Valence Bond vs Molecular Orbital Theory: Key Differences
Description
- VB Theory: Valence bond theory is a molecular theory that is used to define the chemical bonding of atoms in a molecule. Valence bond theory is based on localized bond approach, in which it assumes that the electrons in a molecule occupy atomic orbitals for the individual atoms.
- MO Theory: Molecular orbital theory is a basic theory that is used to define the chemical bonding of a molecule by use of hypothetical molecular orbitals. The molecular orbital theory is a way of looking at the structure of a molecule by using molecular orbitals that belong to the molecule as whole rather than to the individual atoms.
Focus
- VB Theory: Emphasizes the overlap of atomic orbitals between adjacent atoms as the primary factor in bond formation.
- MO Theory: Focuses on the creation of molecular orbitals spanning the entire molecule and treats electrons as distributed throughout these orbitals.
Orbital Overlap
- VB Theory: Relies on the concept of orbital overlap to explain the formation of covalent bonds.
- MO Theory: Forms molecular orbitals through linear combinations of atomic orbitals from all atoms, without requiring direct orbital overlap.
Electron Distribution
- VB Theory: Treats electrons as localized in specific atomic orbitals, leading to the concept of bond pairs and lone pairs.
- MO Theory: Treats electrons as delocalized throughout the entire molecule, represented by molecular orbitals.
Bonding Picture
- VB Theory: Describes bonding in terms of localized electron pairs formed between two atoms.
- MO Theory: Describes bonding in terms of molecular orbitals, encompassing the entire molecule and often involving delocalization of electrons.
Hybridization
- VB Theory: Utilizes hybridization to rationalize molecular geometries and bond angles.
- MO Theory: Generally does not rely on hybridization, as it treats atomic and molecular orbitals in a more continuous manner.
Interaction Energy
- VB Theory: Emphasizes the interaction energy of specific localized orbitals, focusing on the bond itself.
- MO Theory: Considers the entire molecular energy, including both bonding and antibonding interactions.
Complexity of Molecules
- VB Theory: More suitable for simple molecules and situations where localized bonding is dominant.
- MO Theory: Can handle more complex molecules and situations with significant delocalization and resonance.
Resonance
- VB Theory: Explains resonance using a combination of different Lewis structures.
- MO Theory: Handles resonance by considering all possible molecular orbitals and their contributions to the overall electronic structure.
Predictions of Magnetic Properties
- VB Theory: Often less accurate in predicting magnetic properties of molecules due to its focus on localized electron pairs.
- MO Theory: More accurate in predicting magnetic properties as it considers delocalized electron distribution.
Bond Strength and Energy
- VB Theory: Provides qualitative explanations for bond strength and energy by considering overlap and hybridization.
- MO Theory: Offers a more quantitative approach to bond strength and energy calculations through molecular orbital energies and their interactions.
Valence Bond vs Molecular Orbital Theory: Key Takeaways

The document Comparison of Valence Bond and Molecular Orbital Theories | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Comparison of Valence Bond and Molecular Orbital Theories - Chemistry Optional Notes for UPSC
| 1. What is the difference between Valence Bond Theory (VBT) and Molecular Orbital Theory (MOT)? |  |
| 2. What are the applications of Valence Bond Theory? |  |
Ans. Valence Bond Theory is used to explain the formation of covalent bonds, the hybridization of atomic orbitals, and the shape of molecules. It is also used to understand the concept of resonance and the strengths of chemical bonds.
| 3. What are the limitations of Valence Bond Theory? |  |
Ans. Valence Bond Theory has limitations in explaining the magnetic properties of molecules, the shapes of molecules with more than four electron pairs, and the relative stability of molecules. It also does not provide a clear picture of delocalized electrons in conjugated systems.
| 4. What are the applications of Molecular Orbital Theory? |  |
Ans. Molecular Orbital Theory is used to explain the bonding in metal complexes, the stability of aromatic compounds, the concept of bond order and bond strength, and the spectroscopic properties of molecules. It is also used in the study of transition metal complexes and coordination compounds.
| 5. What are the limitations of Molecular Orbital Theory? |  |
Ans. Molecular Orbital Theory has limitations in predicting the exact bond length and bond angle in molecules. It also does not provide a clear picture of the nature of chemical bonding in some molecules, such as transition metal complexes. Additionally, it can be computationally expensive for large molecules.
Related Searches






















