Cell EMF | Chemistry Optional Notes for UPSC PDF Download
Electromotive Force (EMF)
- The electromotive force (EMF) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. This quantity is related to the tendency for an element, a compound or an ion to acquire (i.e. gain) or release (lose) electrons. For example, the maximum potential between Zn and Cu of a well known cell
Zn(s)|Zn2+(1M)||Cu2+(1M)|Cu(s) - has been measured to be 1.100 V. A concentration of 1 M in an ideal solution is defined as the standard condition, and 1.100 V is thus the standard electromotive force, ΔEo, or standard cell potential for the Zn−Cu galvanic cell.
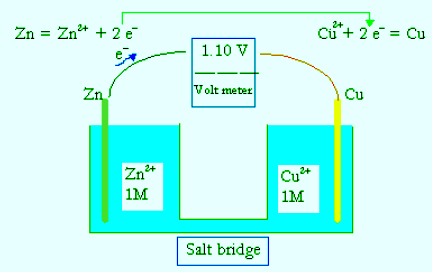
- The standard cell potential, ΔEo, of a galvanic cell can be evaluated from the standard reduction potentials of the two half cells Eo. The reduction potentials are measured against the standard hydrogen electrode (SHE):
Pt(s)|H2(g,1.0atm)|H+ (1.0M) - Its reduction potential or oxidation potential is defined to be exactly zero.
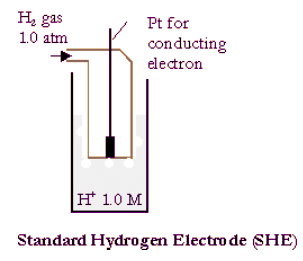
- The reduction potentials of all other half-cells measured in volts against the SHE are the difference in electrical potential energy per coulomb of charge.
- Note that the unit for energy J = Coulomb volt, and the Gibbs free energy G is the product of charge q and potential difference E:
G in J = q E in C V
for electric energy calculations.
Evaluating Standard Cell Potential DE° of Galvanic Cells
- A galvanic cell consists of two half-cells. The convention in writing such a cell is to put the (reduction) cathode on the right-hand side, and the (oxidation) anode on the left-hand side. For example, the cell
Pt|H2|H + ||Zn2+ |Zn
consists of the oxidation and reduction reactions:- H2 → 2e− + 2H+ anode (oxidation) reaction
- Zn2+ + 2e− → Zn cathode (reduction) reaction
- If the concentrations of H+ and Zn2+ ions are 1.0 M and the pressure of H2 is 1.0 atm, the voltage difference between the two electrodes would be -0.763 V(the Zn electrode being the negative electrode). The conditions specified above are called the standard conditions and the EMF so obtained is the standard reduction potential.
- Note that the above cell is in reverse order compared to that given in many textbooks, but this arrangement gives the standard reduction potentialsdirectly, because the Zn half cell is a reduction half-cell. The negative voltage indicates that the reverse chemical reaction is spontaneous. This corresponds to the fact that Zn metal reacts with an acid to produce H2 gas.
As another example, the cell- Pt|H2|H+||Cu+|Cu
- consists of an oxidation and a reduction reaction:
- H2 → 2e− + 2H+ anode reaction
- Cu2+ + 2e− → Cucathode reaction
- and the standard cell potential is 0.337 V. The positive potential indicates a spontaneous reaction,
Cu2+ + H2 → Cu + 2H+
but the potential is so small that the reaction is too slow to be observed.
Solved Example
Example 1: What is the potential for the following cell?
Zn|Zn2+ (1.0M)||Cu2+ (1.0M)| Cu
Ans: From a table of standard reduction potentials we have the following values
Cu2+ + 2e− → CuE∘ = 0.337 (1)
Zn → Zn2+ + 2e−E∗ = 0.763 (2)
Add (1) and (2) to yield
Zn+ Cu2+ → Zn2+ + CuDE∘ = E∘ + E∗ = 1.100 V
Note that E* is the oxidation standard potential, and E° is the reduction standard potential, E* = - E°. The standard cell potential is represented by dE°.
Discussion
The positive potential confirms your observation that zinc metal reacts with cupric ions in solution to produce copper metal.
Example 2: What is the potential for the following cell?
Ag|Ag+ (1.0M)||Li + (1.0M)|Li
Ans: From the table of standard reduction potentials, you find
Li+ + e− → Li E∘ = −3.045 (3)
Ag → Ag+ + e− E∗ = − 0.799 (4)
According to the convention of the cell, the reduction reaction is on the right. The cell on your left-hand side is an oxidation process. Thus, you add (4) and (3) to obtain
Li+ + Ag→Ag+ + Li dE∘ = -3.844 V
Discussion
- The negative potential indicates that the reverse reaction should be spontaneous.
- Some calculators use a lithium battery. The atomic weight of Li is 6.94, much lighter than Zn (65.4).
Summary
- The electromotive force (EMF) is the maximum potential difference between two electrodes of a galvanic or voltaic cell.
- The standard reduction potential of Mn+,1M | M couple is the standard cell potential of the galvanic cell:
Pt|H2,1atm|H+,1M||Mn+, 1M|M - The standard oxidation potential of M|Mn+, 1M couple is the standard cell potential of the galvanic cell:
M|Mn+,1M||H+,1M|H2,1atm|Pt - If the cell potential is negative, the reaction is reversed. In this case, the electrode of the galvanic cell should be written in a reversed order.
FAQs on Cell EMF - Chemistry Optional Notes for UPSC
| 1. What is electromotive force (EMF)? |  |
| 2. How is the standard cell potential (E°) evaluated for galvanic cells? |  |
| 3. What is the significance of evaluating the standard cell potential (E°) of galvanic cells? |  |
| 4. How can the electromotive force (EMF) of a cell be calculated using the standard cell potential (E°)? |  |
| 5. Why is it important to consider the standard cell potential (E°) in galvanic cells? |  |