Neighboring Group Participation | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Heteroatom Nucleophiles as Neighboring Groups |

|
| Solved Examples |

|
| Benzene Ring Pi Bonds as Neighboring Groups |

|
| Alkene Pi Bonds as Neighboring Groups |

|
Heteroatom Nucleophiles as Neighboring Groups
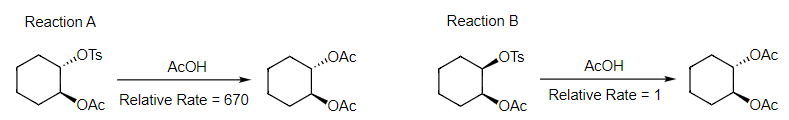
- We have already seen an example of this class of reactions in the preceding chapter. As a reminder, the diastereomeric tosylates react with acetic acid to yield the identical trans diacetate product. (For an explanation of this outcome, including the reaction mechanisms, please revisit Chapter 3.1.)
- We also should note that the relative rates of these reactions vary dramatically, with Reaction A proceeding 670 times faster than Reaction B. This further highlights the power of neighboring group participation; it can dramatically enhance the rate of reactions versus standard nucleophilic substitution reactions. We will see further examples of this throughout the chapter.
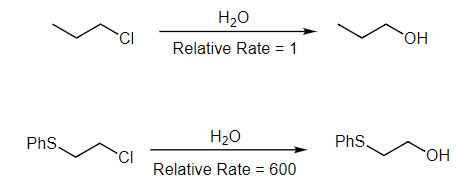
- We can see another NGP example below with the reactions of the different primary alkyl chlorides with water. In the absence of the rate information, we would assume these are both simple SN2 reactions. However, the dramatic differences in reaction rates indicate that something unusual is happening. This is when we should look for a neighboring group, hopefully spotting the sulfur in the bottom reaction.
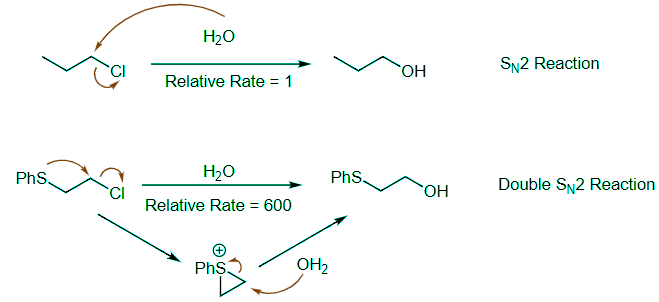
- The S can act as an excellent internal nucleophile and promote an intramolecular reaction. As highlighted below in the mechanisms for these two reactions, the top reaction is a straightforward SN2 reaction. The bottom reaction is much faster because of NGP with the internal S nucleophile. This creates a cyclic sulfonium ion intermediate that reacts much more quickly with water.
Solved Examples
Examples 1: Propose a mechanism to explain the outcome of the following reaction.
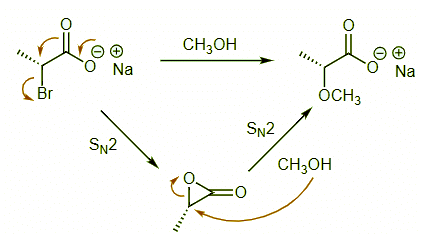
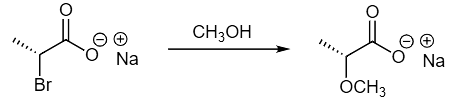 Ans: This is an example of a substitution reaction that proceeds with retention. As we have already seen, this is a hallmark for an NGP mechanism. In this case, the internal nucleophile is a carboxylate that forms an epoxide-type intermediate which reacts quickly with the methanol solvent. These two consecutive SN2 reactions result in overall retention of configuration.
Ans: This is an example of a substitution reaction that proceeds with retention. As we have already seen, this is a hallmark for an NGP mechanism. In this case, the internal nucleophile is a carboxylate that forms an epoxide-type intermediate which reacts quickly with the methanol solvent. These two consecutive SN2 reactions result in overall retention of configuration.
Example 2: Propose a mechanism to explain how both products are formed in the reaction.
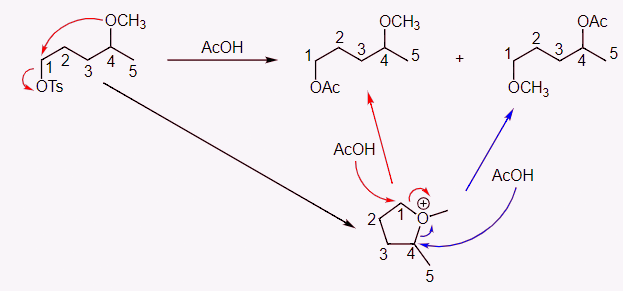
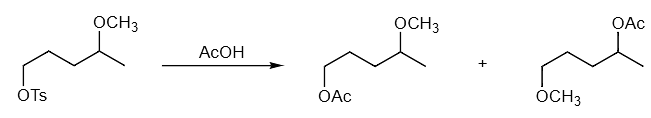 Ans: If this was a simple substitution reaction, we would only form the first product. Seeing that two products are formed, including the second one that looks very strange, we should focus on neighboring group participation. The ether O is a very good internal nucleophile. It can react to form a cationic five-membered ring intermediate. This common intermediate can lead to formation of both of the products depending on which carbon in the intermediate is attacked.
Ans: If this was a simple substitution reaction, we would only form the first product. Seeing that two products are formed, including the second one that looks very strange, we should focus on neighboring group participation. The ether O is a very good internal nucleophile. It can react to form a cationic five-membered ring intermediate. This common intermediate can lead to formation of both of the products depending on which carbon in the intermediate is attacked.
Benzene Ring Pi Bonds as Neighboring Groups
- As demonstrated in the following pair of reactions, benzene ring pi bonds are very effective neighboring groups. As we have already seen, without the rate data, we would not know that anything interesting is happening when comparing these two reactions. How is it possible that the second reaction is 3,000 times faster than the first one? With no heteroatom containing a lone pair, it must be the presence of the benzene ring.
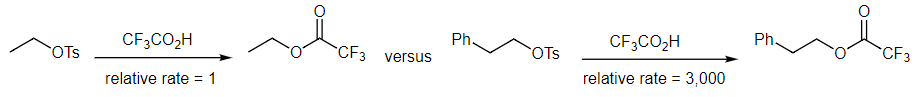
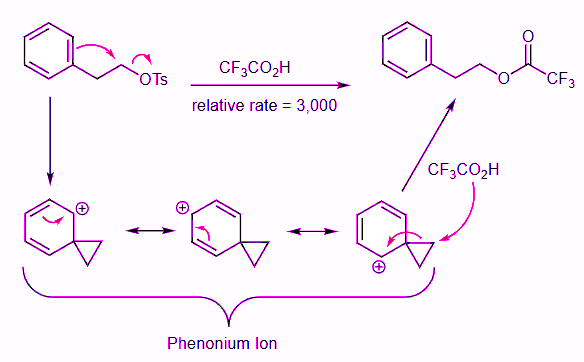
- The first reaction is a standard SN2 reaction while the second one results from two SN2 reactions, as shown in the mechanism below. Like when doing electrophilic aromatic substitution reactions in Intro Orgo, the phenyl ring acts as a nucleophile to displace the tosylate leaving group. This generates a resonance stabilized three-membered ring phenonium ion intermediate. Addition of the trifluoroacetate nucleophile breaks open the three membered ring and restores aromaticity to the benzene ring.
Example 3: Propose a mechanism to explain the results of the following reaction. Note: You are starting with a single enantiomer of the starting material.
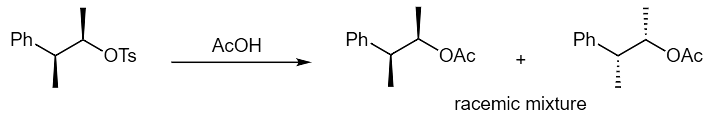
Ans: We can verify this isn't an SN2 reaction by drawing out the product that would result from inversion at the carbon bearing the tosylate. Again, we must look to neighboring group participation. When the benzene ring attacks the tosylate it yields an achiral phenonium ion. The acetate nucleophile can attack either the left or right side of the three membered ring to yield the product with a restored benzene ring. The two products formed are mirror images and flipping the product on the left demonstrates that we do form the target product mixture.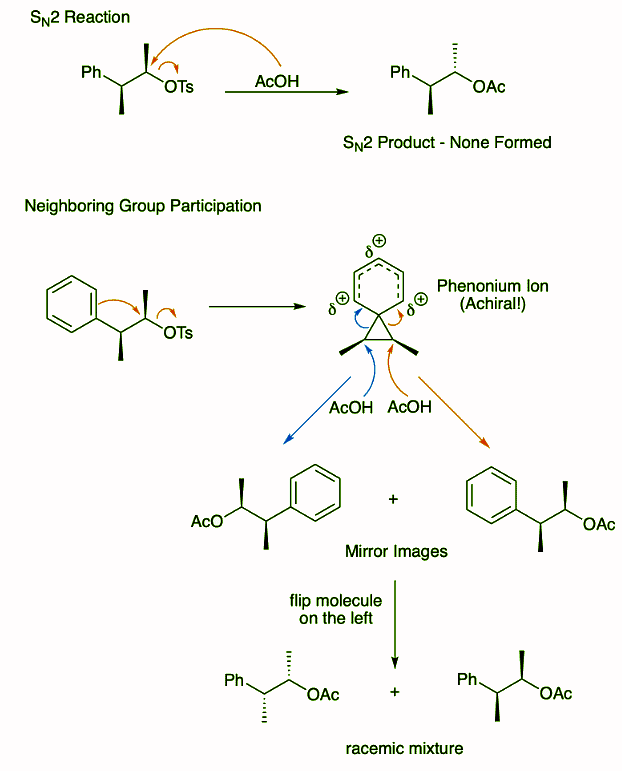
 |
Download the notes
Neighboring Group Participation
|
Download as PDF |
Alkene Pi Bonds as Neighboring Groups
- This is, perhaps, the most interesting example of neighboring group participation. It was definitely the most controversial and involved many research groups who investigated whether the key intermediate was a classical or non-classical carbocation. The principal players in this drama were H.C. Brown (Nobel Prize winner for hydroboration reactions) who favored the classical carbocation and Saul Winstein who promoted the non-classical explanation. Winstein's view was ultimately proven correct based on a variety of investigations including NMR and X-ray crystallography.
- For a detailed explanation of this important historical argument, there is an excellent description in Walling's 1983 paper in Accounts of Chemical Research. So, how do we make this fascinating carbocation and what does it have to do with neighboring group participation? The scheme below illustrate the key reactions, and we will see the carbocation when we illustrate the mechanism. First, to the reactions! The second reaction is a straightforward SN2 reaction. The first reaction has an unbelievable rate enhancement of 1011! This is truly remarkable when compared to the other rate enhancements we've seen in this chapter with the largest being 3,000 for the phenonium ion reaction.
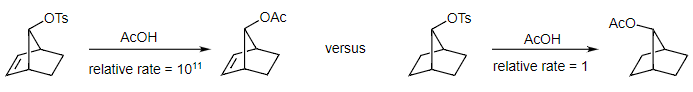
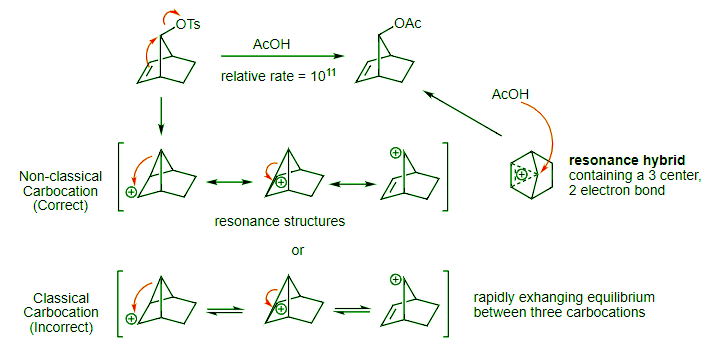
- So, what is going on? This is where we wade into the classical versus non-classical carbocation debate. Clearly, the neighboring alkene pi bond is acting as the neighboring group and pushing off the tosylate leaving group. This is our first SN2 reaction. What is the structure of the cation formed when this happens? We have two options. For both the classical and non-classical carbocation options, the structures look identical. The critical difference is that we are using different types of arrows to connect the structures. For the classical carbocation, we are showing the structures as rapidly interconverting cations that are in equilibrium.
- They are all distinct intermediates. For the non-classical carbocation, these cations are resonance structures, so there are not intermediates at all. Instead, the intermediate is the hybrid structure (pictured in a top down view making it easier to see). This is a very strange resonance hybrid. The three dashed lines indicate that the two electrons from the original pi bond are shared over three carbon atoms, making this an unusual three center, two electron bond! In the second SN2 reaction, acetic acid attacks this resonance hybrid to yield the product.
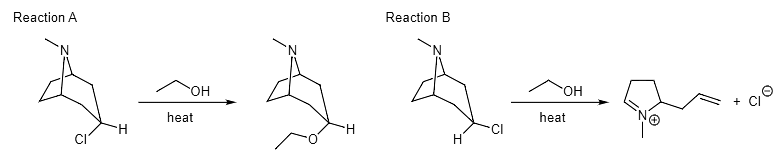
Example 4: The reactions of the isomeric starting materials produce very different products. Propose a mechanism to explain Reaction A. Why can't the starting material in Reaction B undergo a similar reaction? Reaction B is a preview of what we will see in the fragmentation chapter. Propose a mechanism to explain the product formation.
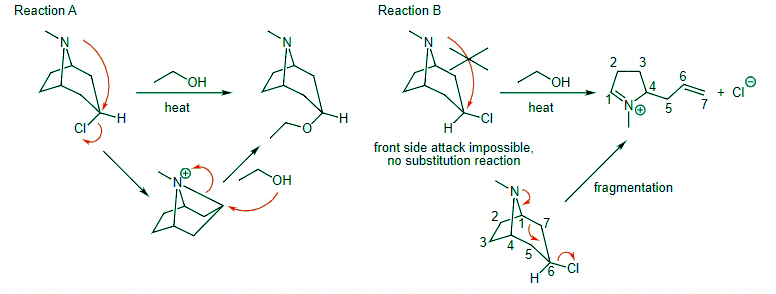
Ans: Reaction A should look familiar. This is another example of neighboring group participation with the N attacking as the internal nucleophile. Two SN2 reactions yield the product that is formed with net retention. In Reaction B, backside attack is impossible because the N is attacking the same face where the leaving group already is. Instead, this molecule fragments, breaking the C1-C7 bond to yield the monocyclic product shown.
FAQs on Neighboring Group Participation - Chemistry Optional Notes for UPSC
| 1. What are heteroatom nucleophiles and how do they act as neighboring groups? |  |
| 2. How do benzene ring pi bonds function as neighboring groups? |  |
| 3. Can alkene pi bonds act as neighboring groups? If yes, how? |  |
| 4. How do neighboring group participation reactions occur? |  |
| 5. Can neighboring group participation be observed in organic synthesis? |  |