Best Study Material for UPSC Exam
UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > SeO2
SeO2 | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Selenium dioxide (SeO2) - Riley oxidation |

|
| Structure & Properties of reagent; Reaction conditions & Workup |

|
| Mechanism |

|
| Applications |

|
Selenium dioxide (SeO2) - Riley oxidation
- Selenium dioxide, SeO2 is an oxidizing agent generally employed in the allylic oxidation of alkenes to furnish allylic alcohols, which may be further oxidized to conjugated aldehydes or ketones. It is also used to oxidize the α-methylene group adjacent to a carbonyl group to give a 1,2-dicarbonyl compound. However selenium dioxide can perform several common types of oxidations, such as alcohols to ketones or aldehydes. The oxidations of methylene groups using Selenium dioxide are referred to as Riley oxidations.
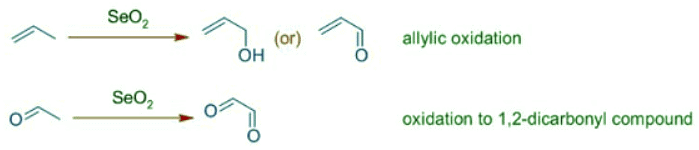 Note: SeO2 is sometimes referred to as selenium(IV) oxide.
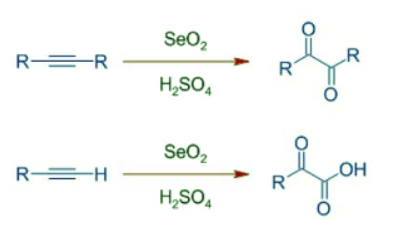
Note: SeO2 is sometimes referred to as selenium(IV) oxide. - Selenium oxide can also be used to oxidize alkynes in presence of acids. The internal alkynes are converted to 1,2-dicarbonyl compounds, whereas terminal alkynes are oxidized to glyoxylic acids.
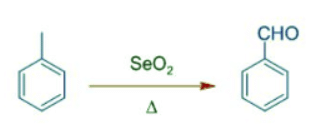
- It also oxidizes benzylic methylene, CH2 group to C=O.
Question for SeO2
Try yourself:
Which of the following is true about selenium dioxide (SeO2)?View Solution
Structure & Properties of reagent; Reaction conditions & Workup
Structure & Properties
- Selenium dioxide is a colorless solid. It exists as one dimensional polymeric chain with alternating selenium and oxygen atoms.
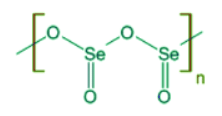
- It sublimes readily and hence the commercial samples of SeO2 can be purified by sublimation.
- SeO2 is an acidic oxide and dissolves in water to form selenous acid, H2SeO3.
Reaction conditions
- Compounds of selenium are very poisonous and smelly. Hence the reaction setup must be maintained under a fuming cupboard. Variety of solvents can be employed.
- Use of acetic acid as solvent stops the reaction at allylic alcohol stage due to formation of acetate esters.
- A convenient way to carry out the reaction is to use only a catalytic amount of SeO2 along with an oxidizing agent like t-butyl hydroperoxide, that reoxidizes the selenium(II) compounds after each cycle of the reaction. This eliminates the need to get rid of large amounts of selenium compounds, which are toxic and usually smelly. It also ensures the principal product is allylic alcohol by reducing the chances of further oxidation to conjugated carbonyl compounds.
Workup
The final workup involves precipitation of selenium or selenium compounds, which can be filtered off before isolation of product from the reaction mixture.
Mechanism
 |
Download the notes
SeO2
|
Download as PDF |
Download as PDF
Allylic oxidation
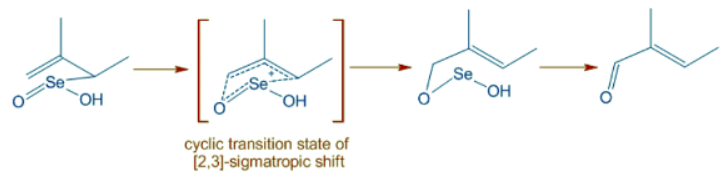
Selenium dioxide oxidizes allylic positions to alcohol or carbonyl groups. It starts with Alder-ene like 4+2 cycloaddition of of SeO2 to give an allylic selenic acid that further undergoes [2,3]-sigmatropic rearrangement to give an unstable compound that may decompose to allylic alcohol or an allylic carbonyl compound as shown below.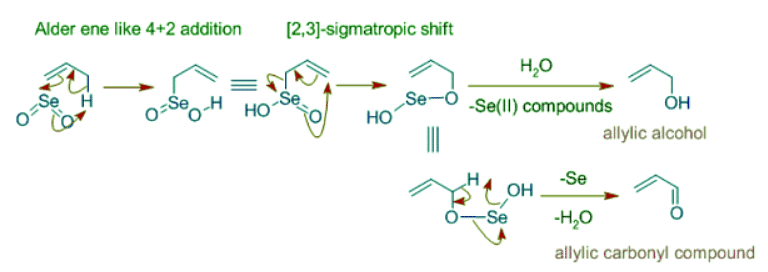
Formation of 1,2-dicarbonyl compounds
SeO2 can also oxidize α-methylene group on a carbonyl compound to furnish 1,2-dicarbonyl compound. The mechanism involves steps similar to allylic oxidation.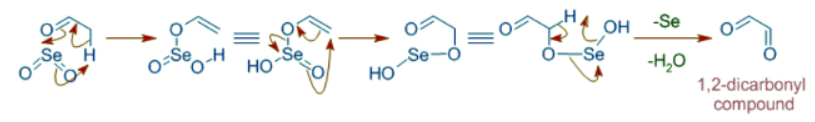
Question for SeO2
Try yourself:
In the allylic oxidation of cyclohexene to cyclohex-2-en-1-ol, which end of the double bond is selectively oxidized?View Solution
Applications
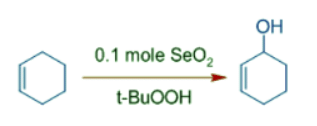
- Catalytic amount of selenium dioxide and t-BuOOH can be employed in the allylic oxidation of cyclohexene to cyclohex-2-en-1-ol, an allylic alcohol.
- Trisubstituted alkenes are oxidized selectively at more substituted end of double bond by giving E-allylic alcohols or conjugated carbonyl compounds predominantly.
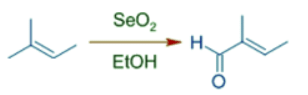
- It is because the initial ene type 4+2 cycloaddition involves preferential attack of the more nucleophilic end of double bond at selenium. In this step, alkene uses the π-HOMO to attack the π*-LUMO of Se=O. Meanwhile the π-HOMO of Se=O attacks the σ*-LUMO of C-H of the allylic system.
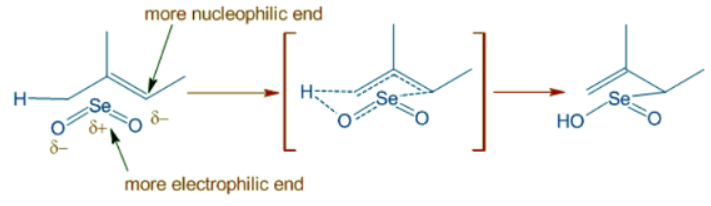
- The E-selectivity is due to cyclic nature of final [2,3] sigmatropic step in which the alkyl substituent adopts pseudoequatorial position.
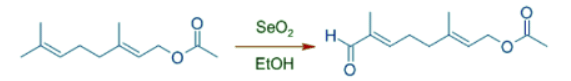
- The allylic oxidation occurs predominantly at most nucleophilic double bond. In the following example, no allylic positions of double bond nearer to electron withdrawing acetyl group are oxidized.
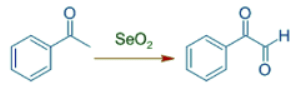
- Acetophenone can be oxidized with SeO2 to oxo(phenyl)acetaldehyde, a 1,2-dicarbonyl compound.
The document SeO2 | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on SeO2 - Chemistry Optional Notes for UPSC
| 1. What is the structure and properties of selenium dioxide (SeO2)? |  |
| 2. What are the reaction conditions and workup for the Riley oxidation using selenium dioxide? |  |
Ans. The Riley oxidation is typically carried out under specific reaction conditions. The reaction requires the presence of selenium dioxide (SeO2) as the oxidizing agent and a suitable solvent such as acetic acid or dichloromethane. The reaction is usually performed at room temperature or slightly elevated temperatures.
After the reaction is complete, the workup involves the removal of excess selenium dioxide and by-products. This is often achieved by adding water or a suitable aqueous solution to the reaction mixture, followed by extraction with an organic solvent. The organic layer is then separated and washed with water to remove any remaining impurities.
| 3. What is the mechanism of the Riley oxidation using selenium dioxide? |  |
Ans. The mechanism of the Riley oxidation involves the transfer of an oxygen atom from selenium dioxide (SeO2) to the substrate being oxidized. The reaction proceeds through a free radical mechanism, where the selenium atom undergoes a redox reaction with the substrate.
In the first step, the selenium atom in SeO2 reacts with a hydrogen atom from the substrate, forming a selenol intermediate. This intermediate then undergoes homolytic cleavage, generating a selenoxyl radical and a radical derived from the substrate.
The selenoxyl radical can then react with another molecule of SeO2, regenerating the selenium atom and forming a peroxy intermediate. This intermediate can further react with the substrate, leading to the oxidation of the substrate and the formation of a new selenoxyl radical.
The process continues until all the desired oxidation products are formed, and the selenium dioxide is consumed in the reaction.
| 4. What are the applications of the Riley oxidation using selenium dioxide? |  |
Ans. The Riley oxidation using selenium dioxide (SeO2) has various applications in organic synthesis. It is commonly used to convert primary alcohols to aldehydes and secondary alcohols to ketones. This oxidation method is particularly useful for selective oxidations, as it allows the conversion of a specific functional group while leaving other groups unaffected.
The Riley oxidation can also be used for the synthesis of various organic compounds, including complex natural products and pharmaceutical intermediates. It provides a mild and efficient method for introducing oxygen functionalities into organic molecules.
| 5. What are the safety precautions to be taken while handling selenium dioxide? |  |
Ans. Selenium dioxide (SeO2) is highly toxic and can pose serious health hazards if not handled properly. Here are some safety precautions to be taken while handling this compound:
1. Use appropriate personal protective equipment (PPE) such as gloves, goggles, and lab coat to prevent direct contact with the skin, eyes, and clothing.
2. Work with selenium dioxide in a well-ventilated area or under a fume hood to avoid inhalation of its vapors or dust particles.
3. Store selenium dioxide in a tightly sealed container in a cool and dry place, away from incompatible substances.
4. Avoid ingestion or ingestion of selenium dioxide. If swallowed or inhaled accidentally, seek immediate medical attention.
5. Dispose of selenium dioxide waste properly according to local regulations and guidelines.
It is essential to follow all necessary safety protocols and consult the Material Safety Data Sheet (MSDS) before working with selenium dioxide.
Related Searches



















