|
|
Vaishnavi VarshneyI am a bsc 2nd year student currently pursuing BSc Hons chemistry and highly interested for pursuing MSc from IIT. |

|
Vaishnavi Varshney
EduRev Chemistry
|
Content, tests & courses saved by you for accessing later. (visible to you only)
Currently preparing for
 | Chemistry |
 | Commerce |
 | Government Jobs |
 | CSIR NET Chemical Science |
Top Scoring Tests by Vaishnavi
Test: VSEPR Theory & shapes of molecules | 20/20 |
Test: Adsorption (Old NCERT) | 40/40 |
Test: Adsorption - 1 | 40/40 |
Test: Molecular Orbital Theory | 20/20 |
Test: Nomenclature Of Organic Compounds | 8/8 |
Test: Hybridisation & Shapes of Molecules | 17/20 |
Test: General Methods of Isolation and Purification - 1 | 17/20 |
Test: Periodic Table of Elements | 17/20 |
Test: Carbon and Boron Family | 17/20 |
Test: Spellings- 4 | 52/60 |
Rated 5  by Vaishnavi
by Vaishnavi
 by Vaishnavi
by VaishnaviDiscussed Questions

|
Vaishnavi Varshney upvoted • Jan 20, 2023 |
Select the compounds which is/are anti-aromatic?- a)
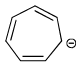
- b)
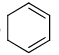
- c)
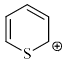
- d)
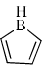
Correct answer is option 'A,D'. Can you explain this answer?
Select the compounds which is/are anti-aromatic?
a)
b)
c)
d)

|
Surbhi Panwar answered |
A is nonaromatic

|
Vaishnavi Varshney asked • Jan 08, 2023 |
The chemical process in the production of steel from haematite ore involve:- a)reduction
- b)oxidation
- c)reduction followed by oxidation
- d)oxidation followed by reduction
Correct answer is option 'C'. Can you explain this answer?
The chemical process in the production of steel from haematite ore involve:
a)
reduction
b)
oxidation
c)
reduction followed by oxidation
d)
oxidation followed by reduction

|
Anshika Chavan answered |
The Production of Steel from Haematite Ore
Haematite ore is a type of iron ore that is commonly used in the production of steel. The chemical process involved in the production of steel from haematite ore involves reduction followed by oxidation.
Reduction
In the first step of the process, haematite ore is heated in the presence of carbon to produce iron and carbon... more
Haematite ore is a type of iron ore that is commonly used in the production of steel. The chemical process involved in the production of steel from haematite ore involves reduction followed by oxidation.
Reduction
In the first step of the process, haematite ore is heated in the presence of carbon to produce iron and carbon... more

|
Vaishnavi Varshney asked • Jan 08, 2023 |
In the commercial electrochemical process for aluminium extraction, the electrolyte used as:- a)Al(OH)3 in NaOH solution
- b)an aqueous solution of Al2(SO4)3
- c)a molten mixture of Al2O3 and Na3AlF6
- d)a molten mixture of AlO(OH) and Al(OH)3
Correct answer is option 'C'. Can you explain this answer?
In the commercial electrochemical process for aluminium extraction, the electrolyte used as:
a)
Al(OH)3 in NaOH solution
b)
an aqueous solution of Al2(SO4)3
c)
a molten mixture of Al2O3 and Na3AlF6
d)
a molten mixture of AlO(OH) and Al(OH)3

|
Madhavan Iyer answered |
Electrochemical Process for Aluminium Extraction
In the commercial electrochemical process for aluminium extraction, the electrolyte used is a molten mixture of Al2O3 and Na3AlF6. Let's understand the process in detail.
Electrolysis of Alumina
The process of extracting aluminium from alumina is called the Hall-Heroult process. It involves the electrolysis of alumin... more
In the commercial electrochemical process for aluminium extraction, the electrolyte used is a molten mixture of Al2O3 and Na3AlF6. Let's understand the process in detail.
Electrolysis of Alumina
The process of extracting aluminium from alumina is called the Hall-Heroult process. It involves the electrolysis of alumin... more

|
Vaishnavi Varshney upvoted • Nov 15, 2022 |
Calculate the surface area of a catalyst that adsorbs 103 cm3 of nitrogen reduced to STP per gram in order to form the monolayer. The effective area occupied by N2 molecule on the surface is 1.62 × 10–15 cm2.
Correct answer is '43416000'. Can you explain this answer?
Calculate the surface area of a catalyst that adsorbs 103 cm3 of nitrogen reduced to STP per gram in order to form the monolayer. The effective area occupied by N2 molecule on the surface is 1.62 × 10–15 cm2.

|
Nepal Dey answered |


|
Vaishnavi Varshney upvoted • Nov 04, 2022 |
According to the crystal field theory, Ni2+ can have two unpaired electron in:- a)Octahedral geo metry only
- b)Tetrahedral geometry only
- c)Square planar geometry only
- d)Both tetrahedral and octahedral geometry
Correct answer is option 'D'. Can you explain this answer?
According to the crystal field theory, Ni2+ can have two unpaired electron in:
a)
Octahedral geo metry only
b)
Tetrahedral geometry only
c)
Square planar geometry only
d)
Both tetrahedral and octahedral geometry

|
Vasanthi Raja answered |
In Ni 2+ it has two unpaired electrons in d orbital in octahedral complex like (Ni(h2o)6) and Nicl4 is tedrahedral complexes the Ni combines with the weak field ligands like halogens, water. so it can't pair up the electrons in Ni2+ , if the nickel combines with strong field ligands such as cyanide it will pair up the electrons in nickel but in undergoes dsp2 geometry and square planar structure... more

|
Vaishnavi Varshney upvoted • Jun 06, 2022 |
Match the Column-I and Column-II:

- a)A-P, B-S, C-R, D-Q
- b)A-R, B-S, C-P, D-Q
- c)A-Q, B-S, C-R, D-P
- d)A-Q, B-S, C-P, D-R
Correct answer is option 'D'. Can you explain this answer?
Match the Column-I and Column-II:

a)
A-P, B-S, C-R, D-Q
b)
A-R, B-S, C-P, D-Q
c)
A-Q, B-S, C-R, D-P
d)
A-Q, B-S, C-P, D-R
|
|
Vedika Singh answered |
Correct Answer :- D
Explanation : A-Q, B-S, C-P, D-R
Type of isomerism which exists between [Pd(C6H5)2(SCN)2] and [Pd(C6H5)2(NCS)2] is: - a)Linkage isomerism
- b)Coordination isomerism
- c)Ionization isomerism
- d)Solvate isomerism
Correct answer is option 'A'. Can you explain this answer?
Type of isomerism which exists between [Pd(C6H5)2(SCN)2] and [Pd(C6H5)2(NCS)2] is:
a)
Linkage isomerism
b)
Coordination isomerism
c)
Ionization isomerism
d)
Solvate isomerism

|
Vaishnavi Varshney answered • May 22, 2022 |
Linkage isomerism because central metal atom is attached with ambident ligand which consists of two donor atoms like NCS
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:- a)1, 5-pentanediol
- b)1, 3-dimethoxypropane
- c)2, 2-dimethyl-1, 3-propanediol
- d)2, 4-pentanediol
Correct answer is option 'C'. Can you explain this answer?
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:
a)
1, 5-pentanediol
b)
1, 3-dimethoxypropane
c)
2, 2-dimethyl-1, 3-propanediol
d)
2, 4-pentanediol

|
Vaishnavi Varshney answered • Mar 23, 2022 |

The 1H NMR spectrum of a compound with molecular formula C3H7NO shows the following features: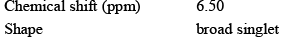
 Which of the following is in agreement with this information:
Which of the following is in agreement with this information:- a)(CH3)2C = NOH
- b)CH3COCH2NH2
- c)CH3CH2CONH2
- d)HCON(CH3)2
Correct answer is option 'C'. Can you explain this answer?
The 1H NMR spectrum of a compound with molecular formula C3H7NO shows the following features:
Which of the following is in agreement with this information:
a)
(CH3)2C = NOH
b)
CH3COCH2NH2
c)
CH3CH2CONH2
d)
HCON(CH3)2

|
Vaishnavi Varshney answered • Mar 21, 2022 |
First count the no of signal ,that should be 3 acc to given ques
also find out the hdi that comes out to be 1 means 1 double bond is present then try to set the multiplicity and delta vlue acc to ques in given options
also find out the hdi that comes out to be 1 means 1 double bond is present then try to set the multiplicity and delta vlue acc to ques in given options
1H NMR spectrum of a mixture of benzene and acetonitrile shows two singlets of equal integration. The molar ratio of benzene: acetonitrile is:
- a)1:1
- b)1:2
- c)2:1
- d)6:1
Correct answer is option 'B'. Can you explain this answer?
1H NMR spectrum of a mixture of benzene and acetonitrile shows two singlets of equal integration. The molar ratio of benzene: acetonitrile is:
a)
1:1
b)
1:2
c)
2:1
d)
6:1

|
Vaishnavi Varshney answered • Mar 21, 2022 |
C should be the answer because of 6:3 equivalent hydrogen in benzene: acetonitrile
Fetching relevant content for you

























