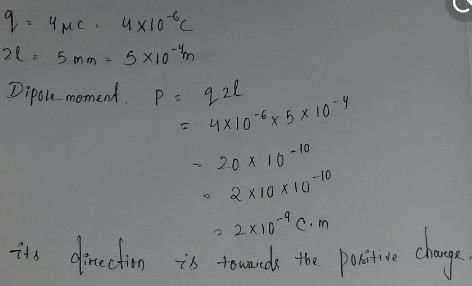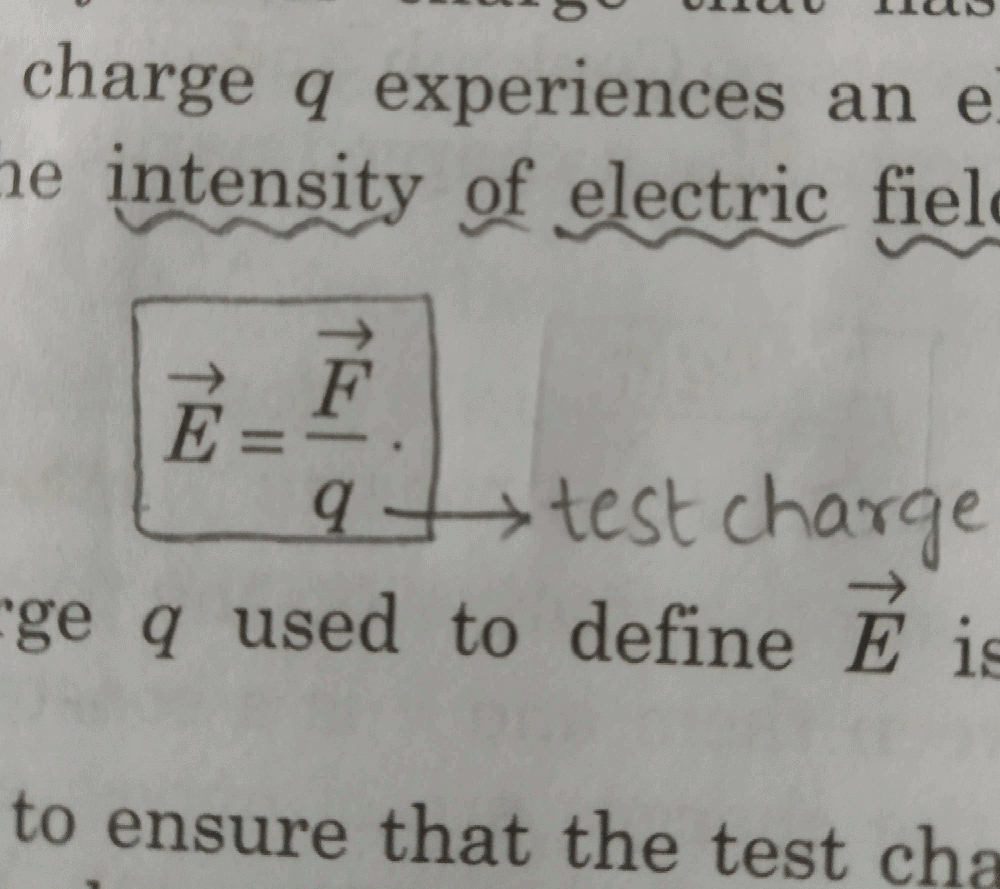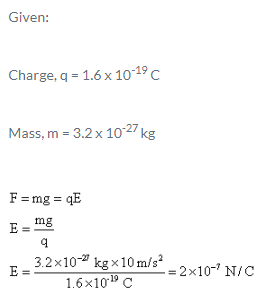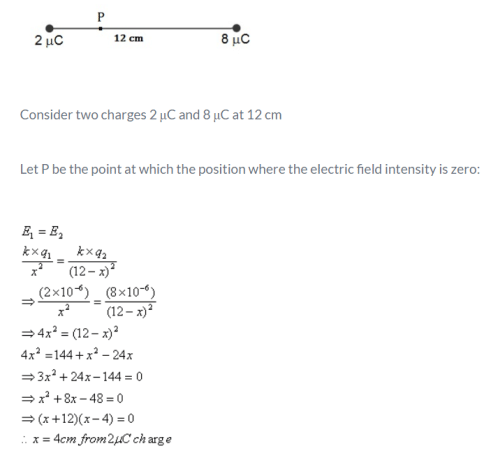All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of April Week 3 for NEET Exam
Eight nucleate embryo sacs are- a)Always tetrasporic
- b)Sometimes monosporic, bisporic and tetrasporic
- c)Always monosporic
- d)Always bisporic
Correct answer is option 'B'. Can you explain this answer?
Eight nucleate embryo sacs are
a)
Always tetrasporic
b)
Sometimes monosporic, bisporic and tetrasporic
c)
Always monosporic
d)
Always bisporic
|
|
Anjali Iyer answered |
Embryo sacs can be divided into three types: monosporic, bisporic, and tetrasporic. In the monosporic, or Polygonum-type embryo sac, meiosis of the diploid megaspore mother cell in the nucellus produces four haploid megaspores.
Fusion of a male gamete with an egg in the embryo sac is called- a)Autogamy
- b)Syngamy
- c)Double fertilisation
- d)Triple fusion
Correct answer is option 'B'. Can you explain this answer?
Fusion of a male gamete with an egg in the embryo sac is called
a)
Autogamy
b)
Syngamy
c)
Double fertilisation
d)
Triple fusion
|
|
Rajat Kapoor answered |
The two male gametes are discharged within the embryo sac. One of the male gamete fuses with the egg cell to form a diploid zygote. This fusion is known as fertilization or syngamy. The second male gamete fuses with the diploid secondary nucleus and forms the triploid Primary Endosperm Nucleus (PEN).
A solution in which no more solute can be dissolved at the given temperature and pressure is called a- a)Unsaturated solution
- b)Dilute solution
- c)Solid solution
- d)Saturated solution
Correct answer is option 'D'. Can you explain this answer?
A solution in which no more solute can be dissolved at the given temperature and pressure is called a
a)
Unsaturated solution
b)
Dilute solution
c)
Solid solution
d)
Saturated solution
|
|
Om Desai answered |
The correct answer is option D
In a saturated solution, more solute cannot be dissolved at a given temperature.
This is because, the solute dissolves in a solvent because of space between particles of solvent but on continuous addition of solute, the space between the solvent particles gets fulfilled. Thus no more solute particle can dissolve in a solvent.
In a saturated solution, more solute cannot be dissolved at a given temperature.
This is because, the solute dissolves in a solvent because of space between particles of solvent but on continuous addition of solute, the space between the solvent particles gets fulfilled. Thus no more solute particle can dissolve in a solvent.
Which is correct about Henry's law- a)The gas in contact with the liquid should behave as an ideal gas
- b)There should not be any chemical interaction between the gas and liquid
- c)The pressure applied should be high
- d)All of these
Correct answer is option 'B'. Can you explain this answer?
Which is correct about Henry's law
a)
The gas in contact with the liquid should behave as an ideal gas
b)
There should not be any chemical interaction between the gas and liquid
c)
The pressure applied should be high
d)
All of these
|
|
Sri Suhas answered |
Yes, because if the gases in the mixture or solution will react then there will be partly solution and partly compound due to which the solution concentration will change and we will not get a proper Henry constant to the solution.
Hope this helps you. If you find an answer to this never hesitate to put it in the answer box.
The diagram shows the electric field lines in a region of space containing two small charged spheres, Y and Z then which statement is true?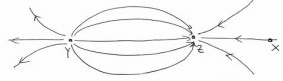
- a)Magnitude of electric field is same everywhere
- b)Y is negative & Z is positive
- c)Y and Z must have the same sign
- d)A small negatively charged body placed at X would be pushed to the right
Correct answer is option 'D'. Can you explain this answer?
The diagram shows the electric field lines in a region of space containing two small charged spheres, Y and Z then which statement is true?
a)
Magnitude of electric field is same everywhere
b)
Y is negative & Z is positive
c)
Y and Z must have the same sign
d)
A small negatively charged body placed at X would be pushed to the right

|
Divey Sethi answered |
A small negatively charged body placed at X would be pushed to the right
The negative charge feels a force opposite to the direction of field.
Other cases are not possible from properties of electric lines of force.
Electric field of a point charge extends up to r where 1/r is- a)one
- b)infinity
- c)Zero
- d)two
Correct answer is option 'C'. Can you explain this answer?
Electric field of a point charge extends up to r where 1/r is
a)
one
b)
infinity
c)
Zero
d)
two

|
Sherlin Dsouza answered |
Hii.... so electric extends upto infinity so inverse of infinity is zero... regards SHERLIN
The field lines for single negative charge are:- a)Radiating outwards
- b)Radiated inwards
- c)Parallel
- d)Spheres concentric with charge
Correct answer is option 'B'. Can you explain this answer?
The field lines for single negative charge are:
a)
Radiating outwards
b)
Radiated inwards
c)
Parallel
d)
Spheres concentric with charge
|
|
Preethi Rajaneekanth answered |
As we know field lines move from positive to negative, when a negative charge is bben considered we can say that the electric field lines move inwards
If electric field lines cross each other that would mean- a)the electric field can be in either of the two directions.
- b)the direction of the field changes at that point
- c)two directions for the electric field at one point which is not possible.
- d)the resultant electric field at a point is zero
Correct answer is option 'C'. Can you explain this answer?
If electric field lines cross each other that would mean
a)
the electric field can be in either of the two directions.
b)
the direction of the field changes at that point
c)
two directions for the electric field at one point which is not possible.
d)
the resultant electric field at a point is zero

|
Sanika Shaikh answered |
Electric field lines can neither be intersect nor be meet it means it has two direction at single point.
For uniform electric field, field lines are:- a)Divergent
- b)Convergent
- c)Parallel and equally spaced
- d)Convergent then divergent
Correct answer is option 'C'. Can you explain this answer?
For uniform electric field, field lines are:
a)
Divergent
b)
Convergent
c)
Parallel and equally spaced
d)
Convergent then divergent
|
|
Preeti Iyer answered |
Uniform field lines imply that every point in space has same magnitude and direction of Electric Field. It is represented by parallel and equally spaced arrows in the direction of electric field
Triploid tissue in angiosperms is- a)Endothecium
- b)Tapetum
- c)Endosperm
- d)Nucellus
Correct answer is option 'C'. Can you explain this answer?
Triploid tissue in angiosperms is
a)
Endothecium
b)
Tapetum
c)
Endosperm
d)
Nucellus

|
Abhilasa Mohapatra answered |
In angiosperms sperm cell fuses with egg to form zygote and another sperm cell fuses with 2 polar nuclei to form endosperm nucleus...so sperm(n)+1 polar nuclei (n)+ 1 polar nuclei (n)=3n
The field lines for single positive charge are:- a)Parallel
- b)Radiating inwards
- c)Circular
- d)Radiating outwards
Correct answer is option 'D'. Can you explain this answer?
The field lines for single positive charge are:
a)
Parallel
b)
Radiating inwards
c)
Circular
d)
Radiating outwards
|
|
Preeti Iyer answered |
Radially outward for +ve charge because it's symmetric. Charges are assumed to be spherical in shape and hence if you apply Gauss law, assuming a spherical Gaussian surface, the electric field MUST be uniformly distributed. Hence they're radial.
Only One Option Correct Type
This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
An unopened soda has an aqueous concentration of C02 at 25° C equal to 0.0506 molal. Thus, pressure of C02 gas in the can is (KH = 0.034 mol/kg bar) - a)0.671 bar
- b)1.49 bar
- c)1.20 bar
- d)1.71 bar
Correct answer is option 'B'. Can you explain this answer?
Only One Option Correct Type
This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
An unopened soda has an aqueous concentration of C02 at 25° C equal to 0.0506 molal. Thus, pressure of C02 gas in the can is (KH = 0.034 mol/kg bar)
a)
0.671 bar
b)
1.49 bar
c)
1.20 bar
d)
1.71 bar
|
|
Abhijeet Sharma answered |
In double fertilisation- a)One male gamete fuses with the egg and the other fuses with the secondary nucleus
- b)One male gamete fuses with the antipodal, while the other fuses with the diploid nucleus
- c)Two male gametes fuse with two eggs
- d)One male gamete fuses with the egg, while the other fuses with the antipodal
Correct answer is option 'A'. Can you explain this answer?
In double fertilisation
a)
One male gamete fuses with the egg and the other fuses with the secondary nucleus
b)
One male gamete fuses with the antipodal, while the other fuses with the diploid nucleus
c)
Two male gametes fuse with two eggs
d)
One male gamete fuses with the egg, while the other fuses with the antipodal
|
|
Subhankar Banerjee answered |
Double fertilisation is a unique process that occurs only in flowering plants. It involves the fusion of two male gametes with two female gametes to produce a zygote and an endosperm. The correct option is (A), one male gamete fuses with the egg and the other fuses with the secondary nucleus.
Process of Double Fertilisation
Double fertilisation occurs in the embryo sac of a flower, which contains the female gametes. The process can be divided into two steps:
First Step: Pollination
During pollination, the male gametes are transferred from the pollen grains to the stigma of the flower. The pollen tube grows down the style and enters the ovary where the embryo sac is located. The pollen tube releases two male gametes into the embryo sac.
Second Step: Fertilisation
In the embryo sac, there are two types of female gametes - the egg and the two polar nuclei. One male gamete fuses with the egg to form a zygote, which will develop into an embryo. The other male gamete fuses with the two polar nuclei to form a triploid nucleus, which will develop into the endosperm.
The Correct Option (A)
In double fertilisation, one male gamete fuses with the egg and the other fuses with the secondary nucleus. The secondary nucleus is also known as the central cell, which contains the two polar nuclei. The fusion of the male gamete with the secondary nucleus forms the triploid nucleus, which develops into the endosperm. The endosperm provides nourishment to the developing embryo.
Conclusion
Double fertilisation is a unique process that occurs only in flowering plants. It involves the fusion of two male gametes with two female gametes to produce a zygote and an endosperm. In double fertilisation, one male gamete fuses with the egg and the other fuses with the secondary nucleus to form the endosperm.
Process of Double Fertilisation
Double fertilisation occurs in the embryo sac of a flower, which contains the female gametes. The process can be divided into two steps:
First Step: Pollination
During pollination, the male gametes are transferred from the pollen grains to the stigma of the flower. The pollen tube grows down the style and enters the ovary where the embryo sac is located. The pollen tube releases two male gametes into the embryo sac.
Second Step: Fertilisation
In the embryo sac, there are two types of female gametes - the egg and the two polar nuclei. One male gamete fuses with the egg to form a zygote, which will develop into an embryo. The other male gamete fuses with the two polar nuclei to form a triploid nucleus, which will develop into the endosperm.
The Correct Option (A)
In double fertilisation, one male gamete fuses with the egg and the other fuses with the secondary nucleus. The secondary nucleus is also known as the central cell, which contains the two polar nuclei. The fusion of the male gamete with the secondary nucleus forms the triploid nucleus, which develops into the endosperm. The endosperm provides nourishment to the developing embryo.
Conclusion
Double fertilisation is a unique process that occurs only in flowering plants. It involves the fusion of two male gametes with two female gametes to produce a zygote and an endosperm. In double fertilisation, one male gamete fuses with the egg and the other fuses with the secondary nucleus to form the endosperm.
Electric field intensity varies with distance as:- a)
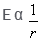
- b)E α r2
- c)
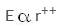
- d)
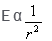
Correct answer is option 'D'. Can you explain this answer?
Electric field intensity varies with distance as:
a)
b)
E α r2
c)
d)

|
Sonu Yadu answered |
It is just like the gravitational force which varies with r^2..
The S.I. unit of electric field intensity is:- a)NC-1
- b)NC
- c)N-1C
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
The S.I. unit of electric field intensity is:
a)
NC-1
b)
NC
c)
N-1C
d)
None of the above
|
|
Krishna Iyer answered |
Electric Field Intensity E = F\q
An electron traveling north enters a region where the electric field is uniform and points North.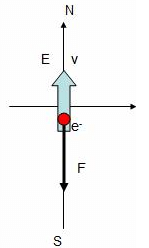
- a)Slows down
- b)Move towards East
- c)Speeds up
- d)Continues with the same speed in the same direction
Correct answer is option 'A'. Can you explain this answer?
An electron traveling north enters a region where the electric field is uniform and points North.
a)
Slows down
b)
Move towards East
c)
Speeds up
d)
Continues with the same speed in the same direction

|
Mohit Rajpoot answered |
Electron is negatively charged and force is always opposite to the direction of electric field. So the when the force acts along South and opposite to the velocity which means the electron slows down.
Electric flux of a surface is maximum, when- a)Surface area is zero.
- b)Electric field is zero.
- c)area vector is perpendicular to the direction of electric field.
- d)area vector is parallel to the direction of electric field.
Correct answer is option 'D'. Can you explain this answer?
Electric flux of a surface is maximum, when
a)
Surface area is zero.
b)
Electric field is zero.
c)
area vector is perpendicular to the direction of electric field.
d)
area vector is parallel to the direction of electric field.

|
Niti Saha answered |
Electric flux is a measure of the electric field passing through a given surface. It is defined as the dot product of the electric field vector and the area vector of the surface. The electric flux through a surface can be calculated using the formula Φ = E ∙ A, where Φ is the electric flux, E is the electric field, and A is the area vector.
To determine the conditions under which the electric flux is maximum, let's analyze the given options:
a) Surface area is zero: If the surface area is zero, then there is no surface for the electric field to pass through. Therefore, the electric flux will be zero.
b) Electric field is zero: If the electric field is zero, then there is no electric field passing through the surface. In this case, the electric flux will also be zero.
c) Area vector is perpendicular to the direction of the electric field: When the area vector is perpendicular to the electric field vector, the dot product of the two vectors will be maximum. This is because the dot product of two vectors is maximum when the vectors are perpendicular to each other. Therefore, in this case, the electric flux will be maximum.
d) Area vector is parallel to the direction of the electric field: When the area vector is parallel to the electric field vector, the dot product of the two vectors will be zero. This is because the dot product of two vectors is zero when the vectors are parallel to each other. Therefore, in this case, the electric flux will be zero.
Hence, the correct answer is option 'd' - the electric flux is maximum when the area vector is parallel to the direction of the electric field.
To determine the conditions under which the electric flux is maximum, let's analyze the given options:
a) Surface area is zero: If the surface area is zero, then there is no surface for the electric field to pass through. Therefore, the electric flux will be zero.
b) Electric field is zero: If the electric field is zero, then there is no electric field passing through the surface. In this case, the electric flux will also be zero.
c) Area vector is perpendicular to the direction of the electric field: When the area vector is perpendicular to the electric field vector, the dot product of the two vectors will be maximum. This is because the dot product of two vectors is maximum when the vectors are perpendicular to each other. Therefore, in this case, the electric flux will be maximum.
d) Area vector is parallel to the direction of the electric field: When the area vector is parallel to the electric field vector, the dot product of the two vectors will be zero. This is because the dot product of two vectors is zero when the vectors are parallel to each other. Therefore, in this case, the electric flux will be zero.
Hence, the correct answer is option 'd' - the electric flux is maximum when the area vector is parallel to the direction of the electric field.
Six charges are kept at the vertices of a regular hexagon as shown in the figure. If magnitude of forceapplied by +Q on +q charge is F, then net electric force on the +Q is:
- a)5F
- b)8F
- c)9F
- d)4F
Correct answer is option 'C'. Can you explain this answer?
Six charges are kept at the vertices of a regular hexagon as shown in the figure. If magnitude of forceapplied by +Q on +q charge is F, then net electric force on the +Q is:

a)
5F
b)
8F
c)
9F
d)
4F

|
New Words answered |
Superposition of Electrostatic Force given by Coulomb’s Law for each of the charge

An electron initially at rest is accelerated through a potential difference of 1 V. The energy gained by the electron is:- a)1 J
- b)10-19J
- c)1.6 × 10-19 J
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
An electron initially at rest is accelerated through a potential difference of 1 V. The energy gained by the electron is:
a)
1 J
b)
10-19J
c)
1.6 × 10-19 J
d)
None of these

|
Surya answered |
Yaa option C is correct... it's a simple easy questionto solve....solution; to find energy... E=q×v q=1.6×10^-19 and V=1 v E=1.6×10^-19×1 E=1.6×10^-19...that's it..hope u clear...!!👍
Concentration of C02 (in mole fraction) in fat when partial pressure of C02 is 55 kPa at 25° C, is (Henry’s law constant of C02 = 8.6 x 104 torr)- a)1.6 x 10-4
- b)4.8 x 10-3
- c)8.6 x 10-4
- d)2.4 x 10-3
Correct answer is option 'B'. Can you explain this answer?
Concentration of C02 (in mole fraction) in fat when partial pressure of C02 is 55 kPa at 25° C, is (Henry’s law constant of C02 = 8.6 x 104 torr)
a)
1.6 x 10-4
b)
4.8 x 10-3
c)
8.6 x 10-4
d)
2.4 x 10-3
|
|
Preethi Kulkarni answered |
Note: Kh (Henry’s law constant) is in pressure unit, hence we use relation, Concentration xKH = Pressure
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)Passage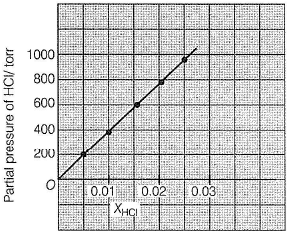 Q.Henry’s law constant for HCI gas is (in torr)
Q.Henry’s law constant for HCI gas is (in torr)- a) 4 x 104
- b) 2x 104
- c)1 x 104
- d)001 x 104
Correct answer is option 'A'. Can you explain this answer?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
Q.
Henry’s law constant for HCI gas is (in torr)
a)
4 x 104
b)
2x 104
c)
1 x 104
d)
001 x 104
|
|
Priyanka Sharma answered |
Based on given figure, pressure of 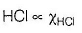
CO(g)is dissolved in H2Oat 25°C and 0.010 atm. Henry’s law constant for this system is 5.80 x104 atm. Thus, mole fraction of CO(g)is- a)1.72 x 10-7
- b)8.28 x 10-7
- c)0.99
- d)0.01
Correct answer is option 'A'. Can you explain this answer?
CO(g)is dissolved in H2Oat 25°C and 0.010 atm. Henry’s law constant for this system is 5.80 x104 atm. Thus, mole fraction of CO(g)is
a)
1.72 x 10-7
b)
8.28 x 10-7
c)
0.99
d)
0.01
|
|
Riya Banerjee answered |
The mole fraction of a gas in solution is related to partial pressure of the gas above the solution by Henry’s law.
The value for Henry’s constant for argon, carbon dioxide, methane and vinyl chloride at 298 K are 40.3 kbar, 1.67 kbar, 0.413 kbar and 0.611 kbar respectively. Which of the gas will be having least solubility?- a)Argon
- b)Vinyl chloride
- c)Methane
- d)Carbon dioxide
Correct answer is option 'A'. Can you explain this answer?
The value for Henry’s constant for argon, carbon dioxide, methane and vinyl chloride at 298 K are 40.3 kbar, 1.67 kbar, 0.413 kbar and 0.611 kbar respectively. Which of the gas will be having least solubility?
a)
Argon
b)
Vinyl chloride
c)
Methane
d)
Carbon dioxide
|
|
Jhanvi Sengupta answered |
The correct answer is Option A
The higher the value of KH, the lower is the solubility of the gas in the liquid. Hence the order of increasing solubility of the gases will be Ar < CO2<CH4< HCHO
The higher the value of KH, the lower is the solubility of the gas in the liquid. Hence the order of increasing solubility of the gases will be Ar < CO2<CH4< HCHO
Henry’s law constant for N2 at 293 K is 76.48 kbar. N2 exerts a partial pressure of 0.987 bar. If N2 gas is bubbled through water at 293 K, then number of millimoles of N2 that will dissolve in 1 L of water is- a)0.0716
- b)1.29 x 10-5
- c)1.29 x 10-2
- d)5.55
Correct answer is option 'A'. Can you explain this answer?
Henry’s law constant for N2 at 293 K is 76.48 kbar. N2 exerts a partial pressure of 0.987 bar. If N2 gas is bubbled through water at 293 K, then number of millimoles of N2 that will dissolve in 1 L of water is
a)
0.0716
b)
1.29 x 10-5
c)
1.29 x 10-2
d)
5.55
|
|
Hansa Sharma answered |
Henry’s law constant is in the unit of pressure, hence we use relation
Let number of moles of nitrogen = n
Which of the following correctly states Gauss law?- a)Electric flux is equal to charge
- b)Electric flux per unit volume is equal to charge
- c)Electric field is equal to charge density
- d)Electric flux per unit volume is equal to volume charge density
Correct answer is option 'D'. Can you explain this answer?
Which of the following correctly states Gauss law?
a)
Electric flux is equal to charge
b)
Electric flux per unit volume is equal to charge
c)
Electric field is equal to charge density
d)
Electric flux per unit volume is equal to volume charge density
|
|
Preeti Iyer answered |
The electric flux passing through any closed surface is equal to the total charge enclosed by that surface. In other words, electric flux per unit volume leaving a point (vanishing small volume), is equal to the volume charge density.
Statement! Soft drinks and soda water (carbonated water) bottles are sealed under high pressure.
Statement II Solubility of C02 increases under high pressure.- a) Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b) Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d) Statement II is correct but Statement I is incorrect
Correct answer is option 'B'. Can you explain this answer?
Statement! Soft drinks and soda water (carbonated water) bottles are sealed under high pressure.
Statement II Solubility of C02 increases under high pressure.
Statement II Solubility of C02 increases under high pressure.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Jhanvi Sengupta answered |
Explanation:
Statement I: Soft drinks and soda water (carbonated water) bottles are sealed under high pressure.
This statement is correct. Soft drinks and soda water are carbonated beverages that contain carbon dioxide (CO2) gas dissolved in water. The carbonation process involves injecting CO2 gas into the liquid under high pressure. The bottles are then tightly sealed to prevent the escape of CO2 gas.
Statement II: Solubility of CO2 increases under high pressure.
This statement is also correct. According to Henry's Law, the solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid. In simple terms, as the pressure increases, the solubility of the gas in the liquid also increases.
Explanation of the relationship between the two statements:
Statement II is the correct explanation of Statement I. The reason soft drinks and soda water bottles are sealed under high pressure is because the solubility of CO2 increases under high pressure. When the bottles are sealed, the high pressure inside the bottle keeps the CO2 gas dissolved in the liquid. If the pressure is released or the bottle is opened, the CO2 gas will come out of the solution, leading to the loss of carbonation and the formation of bubbles.
Therefore, the high pressure is necessary to maintain the carbonation of soft drinks and soda water. The increased solubility of CO2 under high pressure ensures that the gas remains dissolved in the liquid, providing the fizzy and bubbly texture characteristic of carbonated beverages.
Conclusion:
Both Statement I and Statement II are correct, and Statement II is the correct explanation of Statement I. The high pressure at which soft drinks and soda water bottles are sealed is essential for maintaining the carbonation, and the increased solubility of CO2 under high pressure enables the gas to remain dissolved in the liquid.
Statement I: Soft drinks and soda water (carbonated water) bottles are sealed under high pressure.
This statement is correct. Soft drinks and soda water are carbonated beverages that contain carbon dioxide (CO2) gas dissolved in water. The carbonation process involves injecting CO2 gas into the liquid under high pressure. The bottles are then tightly sealed to prevent the escape of CO2 gas.
Statement II: Solubility of CO2 increases under high pressure.
This statement is also correct. According to Henry's Law, the solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid. In simple terms, as the pressure increases, the solubility of the gas in the liquid also increases.
Explanation of the relationship between the two statements:
Statement II is the correct explanation of Statement I. The reason soft drinks and soda water bottles are sealed under high pressure is because the solubility of CO2 increases under high pressure. When the bottles are sealed, the high pressure inside the bottle keeps the CO2 gas dissolved in the liquid. If the pressure is released or the bottle is opened, the CO2 gas will come out of the solution, leading to the loss of carbonation and the formation of bubbles.
Therefore, the high pressure is necessary to maintain the carbonation of soft drinks and soda water. The increased solubility of CO2 under high pressure ensures that the gas remains dissolved in the liquid, providing the fizzy and bubbly texture characteristic of carbonated beverages.
Conclusion:
Both Statement I and Statement II are correct, and Statement II is the correct explanation of Statement I. The high pressure at which soft drinks and soda water bottles are sealed is essential for maintaining the carbonation, and the increased solubility of CO2 under high pressure enables the gas to remain dissolved in the liquid.
Statement I Scuba (instrum ent used for breathing) divers must cope with high concentration of dissolved gases while breathing at high pressure under water.Statement II Formation of bubbles in blood blocks capillaries and creates bends which are painful and dangerous to life.- a) Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b) Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d) Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Statement I Scuba (instrum ent used for breathing) divers must cope with high concentration of dissolved gases while breathing at high pressure under water.
Statement II Formation of bubbles in blood blocks capillaries and creates bends which are painful and dangerous to life.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Prashanth Banerjee answered |
(a) When divers breathe under high pressure, solubility of atmospheric gases in blood increases. When divers come at the surface, pressure gradually decreases. Gases (N2 and 02) are released in the form of bubbles which blocks capillaries and creates a medical symptoms called bends which are painful and dangerous. To avoid bends as well as toxic effects of high concentration of nitrogen in the blood, the tanks used by divers are filled with air diluted with helium (11.7% He, 56.2% N2 and 32.1% Og). Thus, both Statements I and II are correct and Statement II is the correct explanation of Statement I.
Which of the following gases will be least soluble in water?- a)Hydrogen chloride
- b)Sulphur dioxide
- c)Ammonia
- d)Nitrogen
Correct answer is option 'D'. Can you explain this answer?
Which of the following gases will be least soluble in water?
a)
Hydrogen chloride
b)
Sulphur dioxide
c)
Ammonia
d)
Nitrogen
|
|
Ashwin Iyer answered |
Solubility of Gases in Water:
Solubility is the maximum amount of a substance that can dissolve in a given amount of solvent at a specific temperature and pressure. The solubility of a gas in water depends on various factors, such as temperature, pressure, and the chemical nature of the gas.
Factors Affecting Solubility of Gases in Water:
Temperature: The solubility of gases in water decreases with an increase in temperature.
Pressure: The solubility of gases in water increases with an increase in pressure.
Chemical Nature of the Gas: The solubility of gases in water depends on the chemical nature of the gas. Some gases are more soluble in water than others due to their chemical properties.
Least Soluble Gas in Water:
Out of the given options, nitrogen (N2) will be the least soluble gas in water. Nitrogen gas is relatively non-polar, which makes it less likely to dissolve in the polar solvent, such as water. The non-polar nature of nitrogen results in a weak interaction between nitrogen and water molecules, which decreases its solubility.
On the other hand, hydrogen chloride (HCl), sulphur dioxide (SO2), and ammonia (NH3) are more polar and have a stronger interaction with water molecules. Hence, they are more soluble in water than nitrogen gas.
Conclusion:
In conclusion, nitrogen gas will be the least soluble gas in water due to its non-polar nature. While hydrogen chloride, sulphur dioxide, and ammonia are more polar and more soluble in water.
Solubility is the maximum amount of a substance that can dissolve in a given amount of solvent at a specific temperature and pressure. The solubility of a gas in water depends on various factors, such as temperature, pressure, and the chemical nature of the gas.
Factors Affecting Solubility of Gases in Water:
Temperature: The solubility of gases in water decreases with an increase in temperature.
Pressure: The solubility of gases in water increases with an increase in pressure.
Chemical Nature of the Gas: The solubility of gases in water depends on the chemical nature of the gas. Some gases are more soluble in water than others due to their chemical properties.
Least Soluble Gas in Water:
Out of the given options, nitrogen (N2) will be the least soluble gas in water. Nitrogen gas is relatively non-polar, which makes it less likely to dissolve in the polar solvent, such as water. The non-polar nature of nitrogen results in a weak interaction between nitrogen and water molecules, which decreases its solubility.
On the other hand, hydrogen chloride (HCl), sulphur dioxide (SO2), and ammonia (NH3) are more polar and have a stronger interaction with water molecules. Hence, they are more soluble in water than nitrogen gas.
Conclusion:
In conclusion, nitrogen gas will be the least soluble gas in water due to its non-polar nature. While hydrogen chloride, sulphur dioxide, and ammonia are more polar and more soluble in water.
Statement I: People living at high altitudes have symptoms of a condition known as anoxia.Statement II: At high altitudes, the partial pressure of oxygen is less than that at the ground level.- a)Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'B'. Can you explain this answer?
Statement I: People living at high altitudes have symptoms of a condition known as anoxia.
Statement II: At high altitudes, the partial pressure of oxygen is less than that at the ground level.
a)
Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Sounak Mukherjee answered |
At high altitudes, the partial pressure of oxygen is less than that at the ground level. This leads to the low concentration of O2 in the blood and tissues of the people residing in hill areas. This produces weakness and loss of memory.
Symptoms of this conditions is known as ANOXIA. Thus, Statements I are correct but Statement II is the correct explanation of Statement I.
Symptoms of this conditions is known as ANOXIA. Thus, Statements I are correct but Statement II is the correct explanation of Statement I.
Assertion: Soft drinks and soda water bottles are sealed under high pressure.
Reason: High pressure maintains the taste and texture of soft drinks.- a)Assertion is true, but the reason is false.
- b)Both assertion and reason are false.
- c)Both assertion and reason are true. The reason is the correct explanation of assertion.
- d)Both assertion and reason are true. The reason is not the correct explanation of assertion.
Correct answer is option 'A'. Can you explain this answer?
Assertion: Soft drinks and soda water bottles are sealed under high pressure.
Reason: High pressure maintains the taste and texture of soft drinks.
Reason: High pressure maintains the taste and texture of soft drinks.
a)
Assertion is true, but the reason is false.
b)
Both assertion and reason are false.
c)
Both assertion and reason are true. The reason is the correct explanation of assertion.
d)
Both assertion and reason are true. The reason is not the correct explanation of assertion.
|
|
Anirban Joshi answered |
Assertion: Soft drinks and soda water bottles are sealed under high pressure.
Reason: High pressure maintains the taste and texture of soft drinks.
The correct answer is option 'A' - Assertion is true, but the reason is false.
Explanation:
Assertion: Soft drinks and soda water bottles are sealed under high pressure.
When we purchase soft drinks or soda water from the market, we often notice that the bottles are tightly sealed with a cap. This is because soft drinks and soda water are carbonated beverages, which means they contain carbon dioxide gas dissolved in the liquid. The high pressure inside the bottle helps to keep the carbon dioxide dissolved in the liquid, preventing it from escaping and causing the drink to go flat.
When the soft drink or soda water is prepared, carbonation is added by injecting carbon dioxide gas under high pressure into the liquid. The high pressure forces more carbon dioxide to dissolve in the liquid, creating the characteristic fizz and bubbles. In order to maintain this carbonation and prevent the gas from escaping, the bottles are sealed under high pressure.
Reason: High pressure maintains the taste and texture of soft drinks.
This is not a valid reason because the taste and texture of soft drinks are not solely determined by the high pressure inside the bottle. The taste and texture of soft drinks are influenced by various factors such as the ingredients used, the amount of sugar or artificial sweeteners, flavors, and other additives.
While the high pressure inside the bottle helps to maintain the carbonation and prevent the drink from going flat, it does not have a direct impact on the taste and texture of the soft drink. These factors are primarily determined by the formulation and recipe used by the manufacturer.
Therefore, the assertion that soft drinks and soda water bottles are sealed under high pressure is true, but the reason that high pressure maintains the taste and texture of soft drinks is false.
Reason: High pressure maintains the taste and texture of soft drinks.
The correct answer is option 'A' - Assertion is true, but the reason is false.
Explanation:
Assertion: Soft drinks and soda water bottles are sealed under high pressure.
When we purchase soft drinks or soda water from the market, we often notice that the bottles are tightly sealed with a cap. This is because soft drinks and soda water are carbonated beverages, which means they contain carbon dioxide gas dissolved in the liquid. The high pressure inside the bottle helps to keep the carbon dioxide dissolved in the liquid, preventing it from escaping and causing the drink to go flat.
When the soft drink or soda water is prepared, carbonation is added by injecting carbon dioxide gas under high pressure into the liquid. The high pressure forces more carbon dioxide to dissolve in the liquid, creating the characteristic fizz and bubbles. In order to maintain this carbonation and prevent the gas from escaping, the bottles are sealed under high pressure.
Reason: High pressure maintains the taste and texture of soft drinks.
This is not a valid reason because the taste and texture of soft drinks are not solely determined by the high pressure inside the bottle. The taste and texture of soft drinks are influenced by various factors such as the ingredients used, the amount of sugar or artificial sweeteners, flavors, and other additives.
While the high pressure inside the bottle helps to maintain the carbonation and prevent the drink from going flat, it does not have a direct impact on the taste and texture of the soft drink. These factors are primarily determined by the formulation and recipe used by the manufacturer.
Therefore, the assertion that soft drinks and soda water bottles are sealed under high pressure is true, but the reason that high pressure maintains the taste and texture of soft drinks is false.
The value for Henry’s constant for helium, hydrogen, nitrogen and oxygen at 293 K are 144.97 kbar, 69.16 kbar, 76.48 kbar and 34.86 kbar respectively. Which of the gas will be having maximum solubility?- a)Hydrogen
- b)Oxygen
- c)Helium
- d)Nitrogen
Correct answer is option 'B'. Can you explain this answer?
The value for Henry’s constant for helium, hydrogen, nitrogen and oxygen at 293 K are 144.97 kbar, 69.16 kbar, 76.48 kbar and 34.86 kbar respectively. Which of the gas will be having maximum solubility?
a)
Hydrogen
b)
Oxygen
c)
Helium
d)
Nitrogen
|
|
Ritika Sengupta answered |
The correct answer is option B.
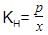
At a constant pressure KH is inversely proportional to x therefore higher the KH lower the solubility.
The increasing solubility order is:
He < N2 < H2 < O2
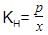
At a constant pressure KH is inversely proportional to x therefore higher the KH lower the solubility.
The increasing solubility order is:
He < N2 < H2 < O2
Select the correct statement(s).- a) KH (Henry’s law constant) is a function of nature of gases
- b) Higher the value of KH at a given pressure, the lower is the solubility of the gas in the liquid
- c) KH increases with increase in temperature
- d) KH increases with increase in partial pressure
Correct answer is option 'A,B,C'. Can you explain this answer?
Select the correct statement(s).
a)
KH (Henry’s law constant) is a function of nature of gases
b)
Higher the value of KH at a given pressure, the lower is the solubility of the gas in the liquid
c)
KH increases with increase in temperature
d)
KH increases with increase in partial pressure
|
|
Isha Rane answered |
(a) At constant temperature, different gases have different KH values. Thus, KH is a function of nature of gases.
Thus, correct.
Thus, correct.
(b) p (partial pressure) = 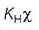
If p is constant and KH value increases then 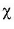 (solubility) decreases. Thus, correct.
(solubility) decreases. Thus, correct.
(c) KH increases with increase in temperature thus, correct.
(d) KH is independent of pressure thus, incorrect.
A handbook lists the solubility of carbon monoxide in water at 0° C and 1 atm pressure as 0.0354 mL CO per mL of H20. What should be the pressure of CO(g) above the solution to obtain 0.010 M CO solution?- a)0.633 atm
- b)6.33 atm
- c)1 atm
- d)0.01 atm
Correct answer is option 'B'. Can you explain this answer?
A handbook lists the solubility of carbon monoxide in water at 0° C and 1 atm pressure as 0.0354 mL CO per mL of H20. What should be the pressure of CO(g) above the solution to obtain 0.010 M CO solution?
a)
0.633 atm
b)
6.33 atm
c)
1 atm
d)
0.01 atm

|
Ashish Nambiar answered |
(b) Volume of CO = 0 .0354 mL = 0 .0354 x 10- 3 L
p = 1 atm, T = 273 K
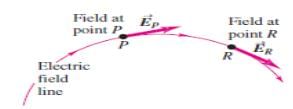
At any point on an electric field line
- a)The perpendicular to the line is in the direction of electric field at that point
- b)The tangent to the line is in the direction of electric field at that point
- c)The normal to the line is in the direction of electric field at that point
- d)The curvature is in the direction of electric field at that point
Correct answer is option 'B'. Can you explain this answer?
At any point on an electric field line
a)
The perpendicular to the line is in the direction of electric field at that point
b)
The tangent to the line is in the direction of electric field at that point
c)
The normal to the line is in the direction of electric field at that point
d)
The curvature is in the direction of electric field at that point
|
|
Nisha Patel answered |
When a tangent is drawn at any point on field line then that tangent gives the direction of electric field at that point
H2S gas is used in qualitative analysis of inorganic cations. Its solubility in water at STP is 0.195 mol kg-1. Thus, Henry’s law constant (in atm molar-1) for H2S is- a)0.195
- b)0.195
- c)3.897 x 103
- d)2.565 x 10-4
Correct answer is option 'B'. Can you explain this answer?
H2S gas is used in qualitative analysis of inorganic cations. Its solubility in water at STP is 0.195 mol kg-1. Thus, Henry’s law constant (in atm molar-1) for H2S is
a)
0.195
b)
0.195
c)
3.897 x 103
d)
2.565 x 10-4
|
|
Shalini Basu answered |
By Henry law:- KHp = s where s = solubility
KH = s/p= 0.195 / 1
= 0.195
KH = s/p= 0.195 / 1
= 0.195
According to Henry’s Law at a constant temperature the solubility of gas in a liquid is directly proportional to the- a)Mass of gas
- b)Density of gas
- c)Volume of gas
- d)Pressure of gas
Correct answer is option 'D'. Can you explain this answer?
According to Henry’s Law at a constant temperature the solubility of gas in a liquid is directly proportional to the
a)
Mass of gas
b)
Density of gas
c)
Volume of gas
d)
Pressure of gas
|
|
Maya Reddy answered |
The correct answer is option D
Henry's law states that at a constant temperature, the solubility of a gas is directly proportional to the pressure of the gas.
In other words, The partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution.
p=KH x
Here, KH is Henry's law constant.
Hence, the given statement is Henry's law.
Henry's law states that at a constant temperature, the solubility of a gas is directly proportional to the pressure of the gas.
In other words, The partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution.
p=KH x
Here, KH is Henry's law constant.
Hence, the given statement is Henry's law.
What is the concentration of 02 in a fresh water stream in equilibrium with air at 25° C and 1.0 bar? Given, KH (Henry’s law constant) of 02 = 1.3 x 10-3 mol/kg bar at 25° C.- a)8.736 x 10-3 g/kg
- b)4.16 x 10-2 g/kg
- c)24.04 g/kg
- d)114.5 g/kg
Correct answer is option 'A'. Can you explain this answer?
What is the concentration of 02 in a fresh water stream in equilibrium with air at 25° C and 1.0 bar? Given, KH (Henry’s law constant) of 02 = 1.3 x 10-3 mol/kg bar at 25° C.
a)
8.736 x 10-3 g/kg
b)
4.16 x 10-2 g/kg
c)
24.04 g/kg
d)
114.5 g/kg

|
Pranjal Pillai answered |
(a) In this case, KH has been given in the unit of mol/kg bar. Thus, solubility of 02 (or concentration) = KH Pgas
In the process of double fertilisation in flowering plants, which of the following statements correctly explains the sequence of events?- a)Pollen tube enters the synergid → Syngamy occurs by the fusion of two male gametes with the egg cell → Triple fusion occurs by the fusion of the zygote with the two polar nuclei.
- b)Pollen tube enters the synergid → One male gamete fuses with the egg cell to form the zygote → The other male gamete fuses with the central cell to form the diploid primary endosperm nucleus (PEN).
- c) Pollen tube enters the synergid → Pollen tube releases two male gametes → One male gamete fuses with the egg cell to form the zygote → The other male gamete fuses with the polar nuclei to form the triploid primary endosperm nucleus (PEN) → Zygote develops into embryo → Primary endosperm nucleus develops into the endosperm.
- d)Pollen tube enters the synergid → One male gamete fuses with the egg cell → The other male gamete fuses with the polar nuclei → Only the zygote is formed.
Correct answer is option 'C'. Can you explain this answer?
a)
Pollen tube enters the synergid → Syngamy occurs by the fusion of two male gametes with the egg cell → Triple fusion occurs by the fusion of the zygote with the two polar nuclei.
b)
Pollen tube enters the synergid → One male gamete fuses with the egg cell to form the zygote → The other male gamete fuses with the central cell to form the diploid primary endosperm nucleus (PEN).
c)
Pollen tube enters the synergid → Pollen tube releases two male gametes → One male gamete fuses with the egg cell to form the zygote → The other male gamete fuses with the polar nuclei to form the triploid primary endosperm nucleus (PEN) → Zygote develops into embryo → Primary endosperm nucleus develops into the endosperm.
d)
Pollen tube enters the synergid → One male gamete fuses with the egg cell → The other male gamete fuses with the polar nuclei → Only the zygote is formed.
|
|
Ayush Chatterjee answered |
Overview of Double Fertilization
Double fertilization is a unique process in flowering plants that involves the fusion of male and female gametes. This process is crucial for the development of the embryo and the endosperm.
Sequence of Events in Double Fertilization
- Pollen Tube Enters the Synergid
The pollen tube penetrates one of the synergid cells located near the egg cell in the ovule.
- Release of Male Gametes
Once inside, the pollen tube releases two male gametes into the female gametophyte.
- Formation of the Zygote
One of the male gametes fuses with the egg cell, resulting in the formation of the diploid zygote, which will develop into the embryo.
- Formation of the Primary Endosperm Nucleus (PEN)
The second male gamete fuses with the two polar nuclei located in the central cell, forming a triploid primary endosperm nucleus (PEN). This structure provides nourishment to the developing embryo.
- Development of Structures
Eventually, the zygote will develop into an embryo, while the primary endosperm nucleus will develop into the endosperm, which serves as a food source for the embryo during its early development stages.
Conclusion
The correct sequence of events in double fertilization is accurately summarized in option 'C'. This option emphasizes the critical roles of both male gametes in forming the zygote and the triploid endosperm, highlighting the unique aspects of flowering plant reproduction.
Double fertilization is a unique process in flowering plants that involves the fusion of male and female gametes. This process is crucial for the development of the embryo and the endosperm.
Sequence of Events in Double Fertilization
- Pollen Tube Enters the Synergid
The pollen tube penetrates one of the synergid cells located near the egg cell in the ovule.
- Release of Male Gametes
Once inside, the pollen tube releases two male gametes into the female gametophyte.
- Formation of the Zygote
One of the male gametes fuses with the egg cell, resulting in the formation of the diploid zygote, which will develop into the embryo.
- Formation of the Primary Endosperm Nucleus (PEN)
The second male gamete fuses with the two polar nuclei located in the central cell, forming a triploid primary endosperm nucleus (PEN). This structure provides nourishment to the developing embryo.
- Development of Structures
Eventually, the zygote will develop into an embryo, while the primary endosperm nucleus will develop into the endosperm, which serves as a food source for the embryo during its early development stages.
Conclusion
The correct sequence of events in double fertilization is accurately summarized in option 'C'. This option emphasizes the critical roles of both male gametes in forming the zygote and the triploid endosperm, highlighting the unique aspects of flowering plant reproduction.
A tennis ball which has been covered with charges is suspended by a thread so that it hangs between two metal plates. One plate is earthed, while other is attracted to a high voltage generator. The ball- a)hangs without moving
- b)is attracted to the high voltage plate and stays there
- c)swings backward & forward hitting each plate in turn
- d)is repelled by earthed plate and stays there
Correct answer is option 'C'. Can you explain this answer?
A tennis ball which has been covered with charges is suspended by a thread so that it hangs between two metal plates. One plate is earthed, while other is attracted to a high voltage generator. The ball
a)
hangs without moving
b)
is attracted to the high voltage plate and stays there
c)
swings backward & forward hitting each plate in turn
d)
is repelled by earthed plate and stays there
|
|
Shubham Jain answered |
The plate which connected to high voltage generator induces negative charge on ball which causes attraction. When the ball strikes the positive plate, charge distribution again takes place that is the bass becomes positive and repulsion takes place. When it strikes the plate which connected to earth than its charge goes to earth and again it will be attracted towards positive plate. Hence the ball swings backward and forward hitting each plate in turn.
Value of Henry’s constant KH is ______.- a)Increases with increase in temperature
- b)Decreases with increase in temperature
- c)Remains constant
- d)First increases, then decreases
Correct answer is option 'A'. Can you explain this answer?
Value of Henry’s constant KH is ______.
a)
Increases with increase in temperature
b)
Decreases with increase in temperature
c)
Remains constant
d)
First increases, then decreases
|
|
Soumya Nair answered |
The correct answer is option A
According to Henry's law, solubility of a gas in a liquid is directly proportional to pressure of gas.
PαX
P = KHX
P = Partial pressure of gas
X = solubility of gas in liquid
KH = Henry's constant
KH depends only on the nature of gas, nature of liquid and temperature (T). As temperature increases, 'KH' increases and 'X' decreases.
PαX
P = KHX
P = Partial pressure of gas
X = solubility of gas in liquid
KH = Henry's constant
KH depends only on the nature of gas, nature of liquid and temperature (T). As temperature increases, 'KH' increases and 'X' decreases.
Statement Type
This section is based on Statement I and Statement II. Select the correct anser from the codes given below Statement I Solubility of gases in liquids decreases with rise in temperature.Statement II CO2(g) + H20 ( / ) ----- » H2CO3(aq ); ΔH = - ve- a) Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b) Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d) Statement II is correct but Statement I is incorrect
Correct answer is option 'B'. Can you explain this answer?
Statement Type
This section is based on Statement I and Statement II. Select the correct anser from the codes given below
This section is based on Statement I and Statement II. Select the correct anser from the codes given below
Statement I Solubility of gases in liquids decreases with rise in temperature.
Statement II CO2(g) + H20 ( / ) ----- » H2CO3(aq ); ΔH = - ve
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Ujwal Patel answered |
(b) Dissolution of gas can be considered as the reverse of vaporisation, i.e. condensation in which heat is evolved. It is thus, exothermic ΔH < 0. By Le-Chatelier’s principle, increase in temperature shifts the dissolution equilibrium in the direction of form ation of C02(g).
Thus, increase in temperature decreases solubility. Thus, both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I.
Thus, increase in temperature decreases solubility. Thus, both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I.
Two point charges QA = 2 μ C and QA = -2 μC located 16 cm apart in vacuum. What is the electric field at the mid point O of the line AB joining the two charges?- a)E = 6.5 mega N/C along OA
- b)E = 6.5 mega N/C along OB
- c)E = 5.6 mega N/C along OB
- d)E = 5.6 mega N/C along OA
Correct answer is option 'C'. Can you explain this answer?
Two point charges QA = 2 μ C and QA = -2 μC located 16 cm apart in vacuum. What is the electric field at the mid point O of the line AB joining the two charges?
a)
E = 6.5 mega N/C along OA
b)
E = 6.5 mega N/C along OB
c)
E = 5.6 mega N/C along OB
d)
E = 5.6 mega N/C along OA

|
Ashwin Yadav answered |
Electric field is the force per unit charge hence, the correct expression among the following is: E=F/Q.
An electric field can deflect- a)X rays
- b)α – rays (He2+)
- c)γ - rays
- d)Neutrons
Correct answer is option 'B'. Can you explain this answer?
An electric field can deflect
a)
X rays
b)
α – rays (He2+)
c)
γ - rays
d)
Neutrons
|
|
Anshika Menon answered |
Only alpha rays are moving with small velocity and having charge so they will be affected by electric field.
X Rays and Gamma Rays are electromagnetic radiations. They do not carry electric charge while neutron is a charge less particle.
X Rays and Gamma Rays are electromagnetic radiations. They do not carry electric charge while neutron is a charge less particle.
Chapter doubts & questions for April Week 3 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of April Week 3 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup