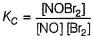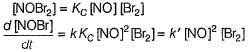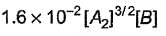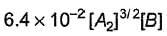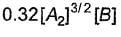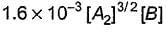All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of July Week 3 for NEET Exam
Two cells of 1.25 V and 0.75V are connected in parallel. The effective voltage will be- a)0.5 V
- b)2.00 V
- c)1.25 V
- d)0.75 V
Correct answer is option 'A'. Can you explain this answer?
Two cells of 1.25 V and 0.75V are connected in parallel. The effective voltage will be
a)
0.5 V
b)
2.00 V
c)
1.25 V
d)
0.75 V
|
|
Anjana Sharma answered |
When two cells are conneted in parallel , then
Effective Voltage,V = V1 − V2 = 1.25 − 0.75 = 0.5 V
At any junction, the sum of the currents entering the junction is equal to the sum of _______- a)potential around any closed loop
- b)voltages across the junction
- c)all the currents in the circuit
- d)currents leaving the junction
Correct answer is option 'D'. Can you explain this answer?
At any junction, the sum of the currents entering the junction is equal to the sum of _______
a)
potential around any closed loop
b)
voltages across the junction
c)
all the currents in the circuit
d)
currents leaving the junction

|
Om Kumar answered |
The correct answer is option 'D': currents leaving the junction.
Explanation:
At any junction in an electrical circuit, the sum of the currents entering the junction is equal to the sum of the currents leaving the junction. This is based on the principle of conservation of charge.
When current flows through a junction, it must split into multiple paths. The total amount of charge entering the junction must be equal to the total amount of charge leaving the junction. This is because charge cannot be created or destroyed, it can only flow through the circuit.
To better understand this concept, consider a simple circuit with three branches connected to a junction. Let's label the currents entering the junction as I1, I2, and I3, and the currents leaving the junction as I4, I5, and I6.
The principle of conservation of charge states that the total amount of charge entering the junction must be equal to the total amount of charge leaving the junction. Mathematically, this can be expressed as:
I1 + I2 + I3 = I4 + I5 + I6
This equation shows that the sum of the currents entering the junction (I1 + I2 + I3) is equal to the sum of the currents leaving the junction (I4 + I5 + I6).
This principle is a consequence of Kirchhoff's current law (KCL), which states that the algebraic sum of currents at any junction in an electrical circuit is zero. This means that the sum of currents entering the junction is equal to the sum of currents leaving the junction.
In summary, at any junction in an electrical circuit, the sum of the currents entering the junction is equal to the sum of the currents leaving the junction. This principle is based on the conservation of charge and is a consequence of Kirchhoff's current law.
Explanation:
At any junction in an electrical circuit, the sum of the currents entering the junction is equal to the sum of the currents leaving the junction. This is based on the principle of conservation of charge.
When current flows through a junction, it must split into multiple paths. The total amount of charge entering the junction must be equal to the total amount of charge leaving the junction. This is because charge cannot be created or destroyed, it can only flow through the circuit.
To better understand this concept, consider a simple circuit with three branches connected to a junction. Let's label the currents entering the junction as I1, I2, and I3, and the currents leaving the junction as I4, I5, and I6.
The principle of conservation of charge states that the total amount of charge entering the junction must be equal to the total amount of charge leaving the junction. Mathematically, this can be expressed as:
I1 + I2 + I3 = I4 + I5 + I6
This equation shows that the sum of the currents entering the junction (I1 + I2 + I3) is equal to the sum of the currents leaving the junction (I4 + I5 + I6).
This principle is a consequence of Kirchhoff's current law (KCL), which states that the algebraic sum of currents at any junction in an electrical circuit is zero. This means that the sum of currents entering the junction is equal to the sum of currents leaving the junction.
In summary, at any junction in an electrical circuit, the sum of the currents entering the junction is equal to the sum of the currents leaving the junction. This principle is based on the conservation of charge and is a consequence of Kirchhoff's current law.
The Wheatstone bridge Principle is deduced using- a)Gauss’s Law
- b)Kirchhoff’s Laws
- c)Coulomb’s Law
- d)Newton’s Laws
Correct answer is option 'B'. Can you explain this answer?
The Wheatstone bridge Principle is deduced using
a)
Gauss’s Law
b)
Kirchhoff’s Laws
c)
Coulomb’s Law
d)
Newton’s Laws
|
|
Anjana Sharma answered |
PRINCIPLE: Wheatstone bridge principle states that when the bridge is balanced, the product of the resistance of the opposite arms are equal. The files that I had attached in which I had derived Wheatstone bridge equation using Kirchhoff law is useful to you.
Can you explain the answer of this question below:The ______ of changes in potential around any closed loop involving resistors and cells in a loop is zero.- A:product
- B:algebraic sum
- C:difference
- D:sum of absolute values
The answer is b.
The ______ of changes in potential around any closed loop involving resistors and cells in a loop is zero.
A:
product
B:
algebraic sum
C:
difference
D:
sum of absolute values
|
|
Lavanya Menon answered |
In accordance with Kirchhoff’s second law i.e. Kirchhoff’s voltage law (KVL), the algebraic sum of all the potential differences in a closed electric circuit or closed loop that contains one or more cells and resistors is always equal to zero.
This law is popularly called the law of conservation of voltage.
This law is popularly called the law of conservation of voltage.
For the reaction, 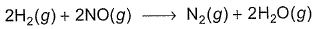
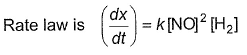 Mechanism is given by
Mechanism is given by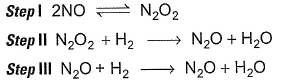 Rate law is true, if
Rate law is true, if - a)step I is the slow step
- b)step II is the slow step
- c)step III is the slow step
- d)step I and step II are the slow steps
Correct answer is option 'B'. Can you explain this answer?
For the reaction, 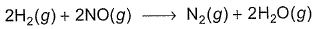
Mechanism is given by
Rate law is true, if
a)
step I is the slow step
b)
step II is the slow step
c)
step III is the slow step
d)
step I and step II are the slow steps

|
Thedot Starry answered |
Yes b is correct because the rate law is written using the slowest step and it matches with second option.
For the reaction, 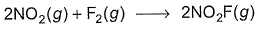 Steps are
Steps are 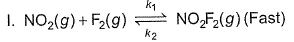
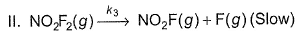
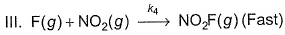
- a)Rate law is in agreement with net stoichiometry of the reaction
- b)Rate law is in agreement with slow step
- c)Rate law is in agreement with net stoichiometry of the reaction as well as with slow step
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
For the reaction, 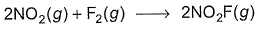
Steps are
a)
Rate law is in agreement with net stoichiometry of the reaction
b)
Rate law is in agreement with slow step
c)
Rate law is in agreement with net stoichiometry of the reaction as well as with slow step
d)
None of the above
|
|
Ananya Singh answered |
Because slow step is the rate determining step
Fused ear lobes appear in the progeny due to an autosomal recessive gene. Work out the genotypes of members in the given pedigree.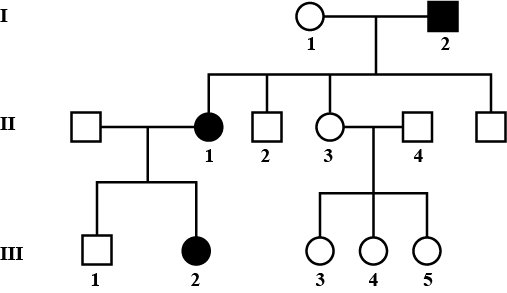
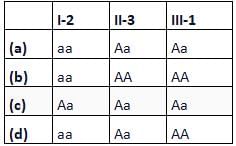
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'D'. Can you explain this answer?
Fused ear lobes appear in the progeny due to an autosomal recessive gene. Work out the genotypes of members in the given pedigree.


a)
a
b)
b
c)
c
d)
d
|
|
Dev Patel answered |
Given pedigree chart for fused ear lobes (autosomal recessive trait) can be explained as follows:
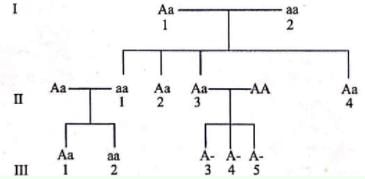
Direction (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.A chemical reaction proceeds through the following steps :Step I, 2A ⇌ X fast
Step II, X+B ⇌ Y slow
Step III, Y+B ⇌ Product fastQ. The rate law for the overall reaction is- a)K'[A]2
- b)K'[B]2
- c)K'[A][B]
- d)K'[A]2[B]
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
A chemical reaction proceeds through the following steps :
Step I, 2A ⇌ X fast
Step II, X+B ⇌ Y slow
Step III, Y+B ⇌ Product fast
Step II, X+B ⇌ Y slow
Step III, Y+B ⇌ Product fast
Q. The rate law for the overall reaction is
a)
K'[A]2
b)
K'[B]2
c)
K'[A][B]
d)
K'[A]2[B]
|
|
Chirag Joshi answered |
By slow step II,
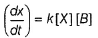
By reversible fast step

∴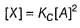
∴
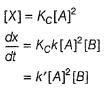
By reversible fast step

∴
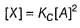
∴
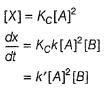
Given N resistors each of resistance R are first combined to get minimum possible resistance and then combined to get maximum possible resistance. The ratio of the minimum to maximum resistance is- a)N
- b)N2
- c)1/N2
- d)1/N
Correct answer is option 'C'. Can you explain this answer?
Given N resistors each of resistance R are first combined to get minimum possible resistance and then combined to get maximum possible resistance. The ratio of the minimum to maximum resistance is
a)
N
b)
N2
c)
1/N2
d)
1/N

|
Nishtha Bose answered |
They are connected in series to get maximum in this case resistance would be nr
and to get minimun resistance they are connected in parallel :. resistance in this case is n/r
:. ratio between minimum and maximum resistance is n/r/nr = 1/r^2
Which one is the incorrect match?- a)

- b)

- c)
- d)

Correct answer is option 'C'. Can you explain this answer?
Which one is the incorrect match?
a)

b)

c)
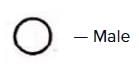
d)


|
Ramya S answered |
It denotes female
males have square shape ◼️
males have square shape ◼️
Select the incorrect statement regarding pedigree analysis- a)Solid symbols show unaffected individuals
- b)Proband is the person from which case history starts
- c)It is useful for genetic counsellors
- d)It is an alalysis of traits in several generations of a family
Correct answer is option 'A'. Can you explain this answer?
Select the incorrect statement regarding pedigree analysis
a)
Solid symbols show unaffected individuals
b)
Proband is the person from which case history starts
c)
It is useful for genetic counsellors
d)
It is an alalysis of traits in several generations of a family
|
|
Ajay Yadav answered |
In pedigree analysis, solid symbol shows affected individuals.
For the reaction,A + 2B → C+ DThe rate law is k [A] [B] .
Select the correct statement.
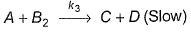
- a)Following mechanism is consistent with the stoichiometry of the net reaction and with the rate lawA + B → I (Slow)I+B → C+D (Fast)
- b)Following mechanism is consistent with the stoichiometry of the net reaction but not with the rate law
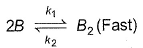
- c)Both (a) and (b)
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
For the reaction,
A + 2B → C+ D
The rate law is k [A] [B] .
Select the correct statement.
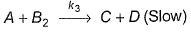
Select the correct statement.
a)
Following mechanism is consistent with the stoichiometry of the net reaction and with the rate law
A + B → I (Slow)
I+B → C+D (Fast)
b)
Following mechanism is consistent with the stoichiometry of the net reaction but not with the rate law
c)
Both (a) and (b)
d)
None of the above

|
Vandana Menon answered |
(a) Slow step is the rate-determining step, thus by
rate law = k[A][B]
Net reaction,

Thus, this mechanism is not consistent with the stoichiometry, but is consistent with rate law.
(b) By slow step,
Rate law = k3[A][B2]
By fast step,
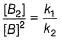
∴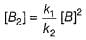
∴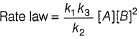
Thus, this mechanism is consistent with the stoichiometry of the net reaction but not with the rate law.
Thus, (b) is correct.
rate law = k[A][B]
Net reaction,

Thus, this mechanism is not consistent with the stoichiometry, but is consistent with rate law.
(b) By slow step,
Rate law = k3[A][B2]
By fast step,
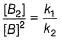
∴
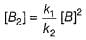
∴
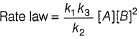
Thus, this mechanism is consistent with the stoichiometry of the net reaction but not with the rate law.
Thus, (b) is correct.
Based on the following steps: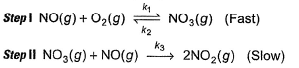 Rate law is
Rate law is - a)
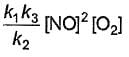
- b)
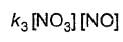
- c)
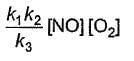
- d)
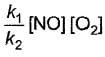
Correct answer is option 'A'. Can you explain this answer?
Based on the following steps:
Rate law is
a)
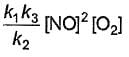
b)
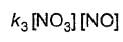
c)
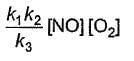
d)
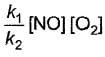

|
Amrutha Pillai answered |
Slow step (II) is the rate-determining step.
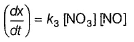
By fast step I in equilibrium.
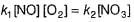
By fast step I in equilibrium.
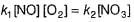
∴ 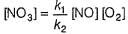
∴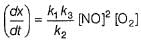
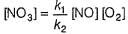
∴
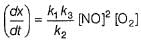
A chemical reaction is said to take place through the various stages with ΔG° values indicated by the graph: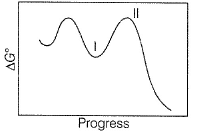 Stages I and II represent respectively
Stages I and II represent respectively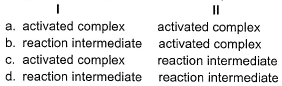
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
A chemical reaction is said to take place through the various stages with ΔG° values indicated by the graph:
Stages I and II represent respectively
a)
a
b)
b
c)
c
d)
d
|
|
Nisha Kulkarni answered |
I is reaction intermediate while II is transition complex.
Inheritance of which of the following traits is shown in the given cross?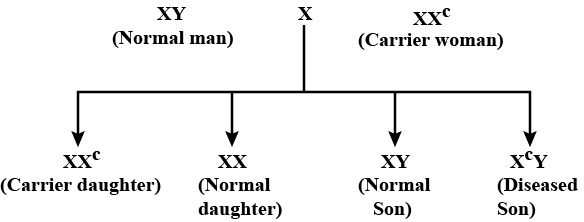
- a)X-linked dominant trait
- b)X-linked recessive trait
- c)Autosomal recessive trait
- d)Autosomal dominant trait
Correct answer is option 'B'. Can you explain this answer?
Inheritance of which of the following traits is shown in the given cross?

a)
X-linked dominant trait
b)
X-linked recessive trait
c)
Autosomal recessive trait
d)
Autosomal dominant trait
|
|
Suresh Iyer answered |
- In the given cross, passing of disease is from carrier female to male progeny (criss-cross inheritance). Any trait that shows criss-cross inheritance is located on the sex chromosome.
- Presence of a single recessive gene i.e. Xc in carrier individuals (XXC) does not cause the disease, thus the trait is recessive.
In the following pedigree chart, the mutant trait is shaded black. The gene responsible for the trait is 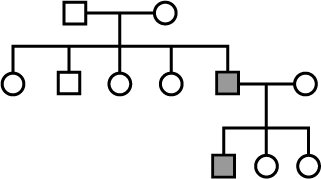
- a)dominant and sex linked
- b)dominant and autosomal
- c)recessive and sex linked
- d)recessive and autosomal
Correct answer is option 'D'. Can you explain this answer?
In the following pedigree chart, the mutant trait is shaded black. The gene responsible for the trait is

a)
dominant and sex linked
b)
dominant and autosomal
c)
recessive and sex linked
d)
recessive and autosomal
|
|
Anjali Sharma answered |
The given trait cannot be sex-linked as sex-linked traits follow criss-cross inheritance and in the given pedigree, no criss-cross inheritance is being followed. The trait exhibited in pedigree chart is autosomal recessive. An autosomal recessive trait appears in case of marriage between two heterozygous individuals (Aa x Aa=3Aa+1aa), a recessive individual with hybrid (Aa x aa=2Aa+2aa) and two recessives (aa x aa= all aa). It expresses its effect only in pure or homozygous state.
If the trait had been controlled by dominant gene, then one of the parent must have possessed the dominant gene and hence the disease.
Direction (Q. Nos. 21 and 22) This section contains 2 questions. When worked out will result in one integer from 0 to 9 (both inclusive).Consider the following proposed mechanism: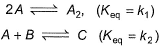
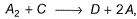 (rate constant =k ) to describe the overall equations
(rate constant =k ) to describe the overall equations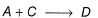 Q.What is the order predicted by the proposed mechanism?
Q.What is the order predicted by the proposed mechanism?
Correct answer is '4'. Can you explain this answer?
Direction (Q. Nos. 21 and 22) This section contains 2 questions. When worked out will result in one integer from 0 to 9 (both inclusive).
Consider the following proposed mechanism:
Q.
What is the order predicted by the proposed mechanism?

|
Aarya Dasgupta answered |
Rate determining step is 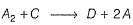
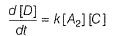


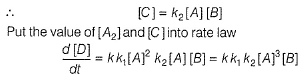
The reaction is third order in A and first order in B.
Total order = 3 + 1 = 4
The reaction is third order in A and first order in B.
Total order = 3 + 1 = 4
Passage III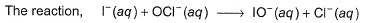 is believed to proceed through the following three step mechanism:
is believed to proceed through the following three step mechanism:
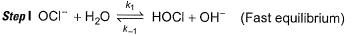
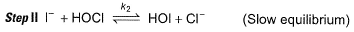
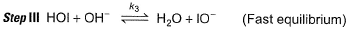 Q.Identify the false statement.
Q.Identify the false statement.- a)The molecularity of each step is 2
- b)The limiting step is II
- c)HOCI is only the reaction intermediate
- d)The reaction is a complex reaction
Correct answer is option 'C'. Can you explain this answer?
Passage III
is believed to proceed through the following three step mechanism:
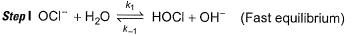
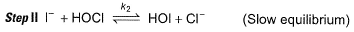
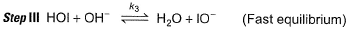
Q.
Identify the false statement.
a)
The molecularity of each step is 2
b)
The limiting step is II
c)
HOCI is only the reaction intermediate
d)
The reaction is a complex reaction

|
Anirudh Gupta answered |
Molecularity of each step is two thus, true.
Thus, (a) is correct.
The limiting step of a complex reaction is always the slowest, thus (b) is correct.
It involves more than one elementary step, thus, it is a complex reaction. Thus, (d) is also correct.
HOCl appears in step I and disappears in step II, Thus it is an intermediate.
HOI appears in step II and disappears in step III. Thus, HOI is also an intermediate.
Thus, (c) is incorrect.
Slow step il is the rate-determining step.
Thus, (a) is correct.
The limiting step of a complex reaction is always the slowest, thus (b) is correct.
It involves more than one elementary step, thus, it is a complex reaction. Thus, (d) is also correct.
HOCl appears in step I and disappears in step II, Thus it is an intermediate.
HOI appears in step II and disappears in step III. Thus, HOI is also an intermediate.
Thus, (c) is incorrect.
Slow step il is the rate-determining step.
Note H2O as reactant is not taken in equilibrium and rate expression.
Passage III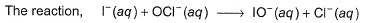 is believed to proceed through the following three step mechanism:
is believed to proceed through the following three step mechanism:
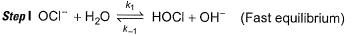
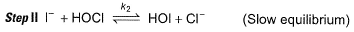
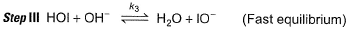 Q.What is the rate equation of the reaction?
Q.What is the rate equation of the reaction?- a)
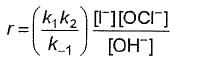
- b)
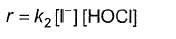
- c)
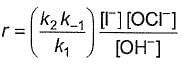
- d)
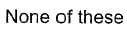
Correct answer is option 'A'. Can you explain this answer?
Passage III
is believed to proceed through the following three step mechanism:
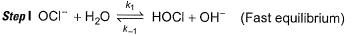
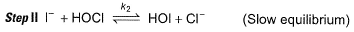
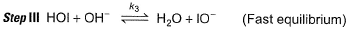
Q.
What is the rate equation of the reaction?
a)
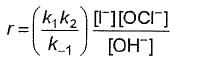
b)
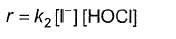
c)
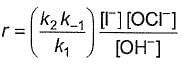
d)

|
Om Kumar answered |
Molecularity of each step is two thus, true.
Thus, (a) is correct.
The limiting step of a complex reaction is always the slowest, thus (b) is correct.
It involves more than one elementary step, thus, it is a complex reaction. Thus, (d) is also correct.
HOCi appears in step I and disappears in step II, Thus it is an intermediate.
HOI appears in step II and disappears in step III. Thus, HOI is also an intermediate.
Thus, (c) is incorrect.
Slow step il is the rate-determining step.
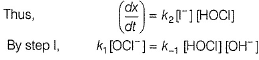
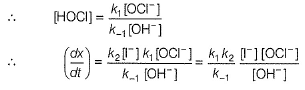
Note H2O as reactant is not taken in equilibrium and rate expression.
Thus, (a) is correct.
The limiting step of a complex reaction is always the slowest, thus (b) is correct.
It involves more than one elementary step, thus, it is a complex reaction. Thus, (d) is also correct.
HOCi appears in step I and disappears in step II, Thus it is an intermediate.
HOI appears in step II and disappears in step III. Thus, HOI is also an intermediate.
Thus, (c) is incorrect.
Slow step il is the rate-determining step.
Note H2O as reactant is not taken in equilibrium and rate expression.
Direction (Q. Nos. 15- 20) This section contains 3 paragraphs, each describing theory, experiments, data, etc., Six questions related to the paragraphs have been given. Each question has ONLY ONE correct answer among the four given options (a), (b), (c) and (d).Passage IThe ozone in the earth’s ozone layer decomposes according to the equation, It involves
It involves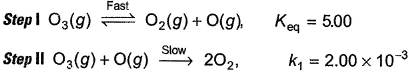 (Time is in seconds and concentration in mol L-1)Q. Rate of disappearance of O3 is
(Time is in seconds and concentration in mol L-1)Q. Rate of disappearance of O3 is- a)
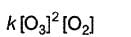
- b)

- c)
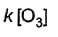
- d)
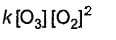
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 15- 20) This section contains 3 paragraphs, each describing theory, experiments, data, etc., Six questions related to the paragraphs have been given. Each question has ONLY ONE correct answer among the four given options (a), (b), (c) and (d).
Passage I
The ozone in the earth’s ozone layer decomposes according to the equation,
It involves
(Time is in seconds and concentration in mol L-1)
Q.
Rate of disappearance of O3 is
a)
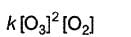
b)

c)
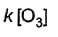
d)
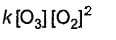
|
|
Sankar Banerjee answered |
Step / is fast and reversible
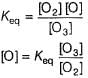
Step // is slow and rate-determining step.
∴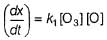
∴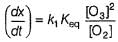
∴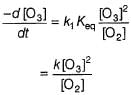
where, k1 keq
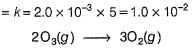
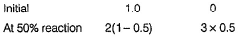
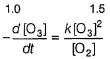
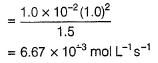
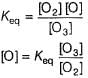
Step // is slow and rate-determining step.
∴
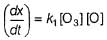
∴
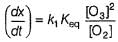
∴
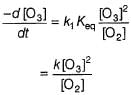
where, k1 keq
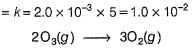
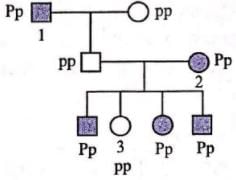
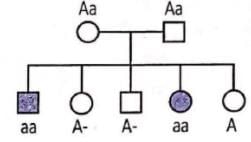
In humans, polydactyly (i.e, presence of extra fingers and toes) is determined by a dominant autosomal allele (P) and the normal condition is determined by a recessive allele (p). Find out the possible genotypes of family members 1, 2 and 3 in the given pedigree.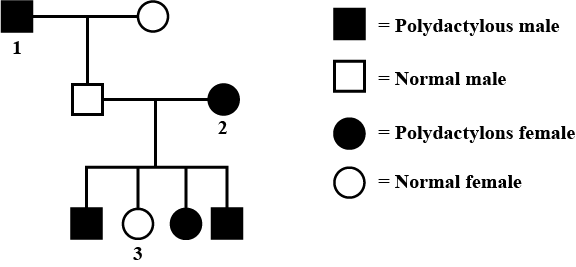 Codes are:
Codes are: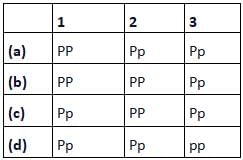
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'D'. Can you explain this answer?
In humans, polydactyly (i.e, presence of extra fingers and toes) is determined by a dominant autosomal allele (P) and the normal condition is determined by a recessive allele (p). Find out the possible genotypes of family members 1, 2 and 3 in the given pedigree.

Codes are:

a)
a
b)
b
c)
c
d)
d
|
|
Jyoti Sengupta answered |
Given pedigree chart for polydactyly (autosomal dominant trait) can be explained as follows :
If A = normal allele, a = albino allele, then genotypes of father and mother respectively are?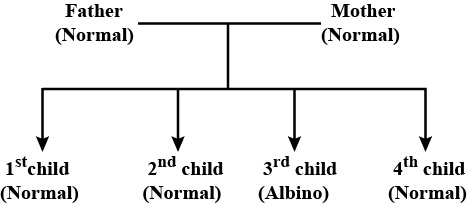
- a)Aa and Aa
- b)AA and Aa
- c)Aa and AA
- d)Aa and aa
Correct answer is option 'A'. Can you explain this answer?
If A = normal allele, a = albino allele, then genotypes of father and mother respectively are?

a)
Aa and Aa
b)
AA and Aa
c)
Aa and AA
d)
Aa and aa
|
|
Jyoti Sengupta answered |
Albinism is hereditary lack of pigmentation in an organism. It is an autosomal recessive disorder. Albino humans lack dark pigment melanin in their skin, hair or eyes. The allele responsible is recessive to allele for normal pigmentation. Father's and mother's genotype should be Aa. They both are carriers of the disease.

The chances of this couple's fifth child being an albino are 1 in 4.
Reaction between nitric oxide and hydrogen takes place by the following mechanism: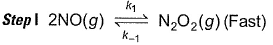
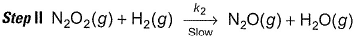
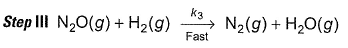 Q.Rate of the overall reaction in terms of the concentration of the reactants is
Q.Rate of the overall reaction in terms of the concentration of the reactants is - a)
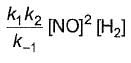
- b)
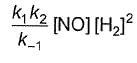
- c)
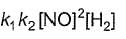
- d)
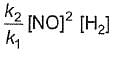
Correct answer is option 'A'. Can you explain this answer?
Reaction between nitric oxide and hydrogen takes place by the following mechanism:
Q.
Rate of the overall reaction in terms of the concentration of the reactants is
a)
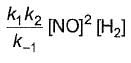
b)
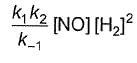
c)
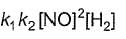
d)
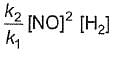

|
Ashwin Yadav answered |
Slow step II is the rate-determining step.
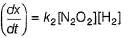 ...(1)
...(1)
By step I in equilibrium,
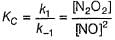
∴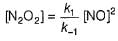
Put the value of [N2O2] in Eq. (i), we get
∴
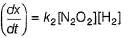 ...(1)
...(1)By step I in equilibrium,
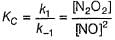
∴
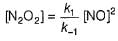
Put the value of [N2O2] in Eq. (i), we get
∴

Passage IThe ozone in the earth’s ozone layer decomposes according to the equation, It involves
It involves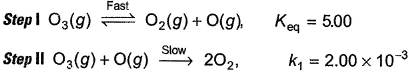 (Time is in seconds and concentration in mol L-1)Q.What is the rate at a time of 50% decomposition of O3, when [O3]0 = 1.0 M?
(Time is in seconds and concentration in mol L-1)Q.What is the rate at a time of 50% decomposition of O3, when [O3]0 = 1.0 M?- a)
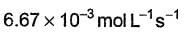
- b)
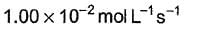
- c)
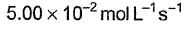
- d)
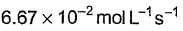
Correct answer is option 'A'. Can you explain this answer?
Passage I
The ozone in the earth’s ozone layer decomposes according to the equation,
It involves
(Time is in seconds and concentration in mol L-1)
Q.
What is the rate at a time of 50% decomposition of O3, when [O3]0 = 1.0 M?
a)
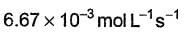
b)
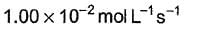
c)
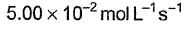
d)
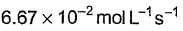

|
Aravind Mehra answered |
Step / is fast and reversible
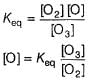
Step // is slow and rate-determining step.
∴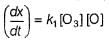
∴
∴
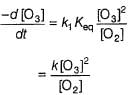
where,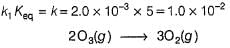
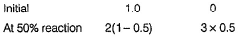
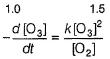
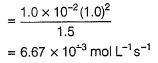
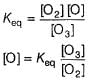
Step // is slow and rate-determining step.
∴
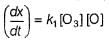
∴

∴
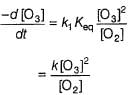
where,
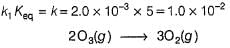
Passage IIN2O5 decomposes according to the following equation: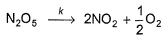 The following mechanism has been given:
The following mechanism has been given: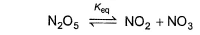
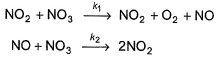 Q.Half-life period is independent of the concentration of N2O5. Thus,
Q.Half-life period is independent of the concentration of N2O5. Thus,- a)
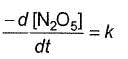
- b)
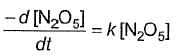
- c)
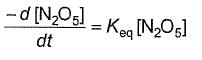
- d)
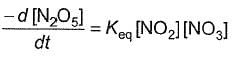
Correct answer is option 'B'. Can you explain this answer?
Passage II
N2O5 decomposes according to the following equation:
The following mechanism has been given:
Q.
Half-life period is independent of the concentration of N2O5. Thus,
a)
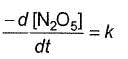
b)
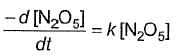
c)
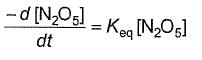
d)
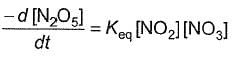

|
Ashwin Yadav answered |
Half-life period is independent of [N2O5]. Hence, it is first order reaction.
Consider the following reaction,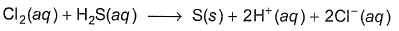 The rate equation for this reaction is, rate = k[CI2] [H2S]
The rate equation for this reaction is, rate = k[CI2] [H2S]
Which of these mechanisms is/are consistent with this rate equation?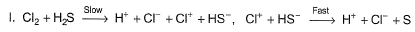
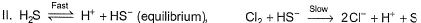 [AIEEE2010]
[AIEEE2010]- a)Only II
- b)Only I
- c)Both (a) and (b)
- d)Neither (a) nor (b)
Correct answer is option 'B'. Can you explain this answer?
Consider the following reaction,
The rate equation for this reaction is, rate = k[CI2] [H2S]
Which of these mechanisms is/are consistent with this rate equation?
Which of these mechanisms is/are consistent with this rate equation?
[AIEEE2010]
a)
Only II
b)
Only I
c)
Both (a) and (b)
d)
Neither (a) nor (b)

|
Anirudh Gupta answered |
Slow step is the rate-determining step.
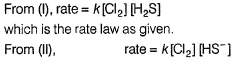
and
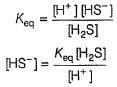
∴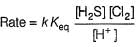
which is not the rate law.
and
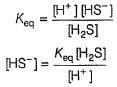
∴
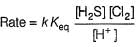
which is not the rate law.
Study the pedigree chart of a family showing the inheritance of myotonic dsytrophy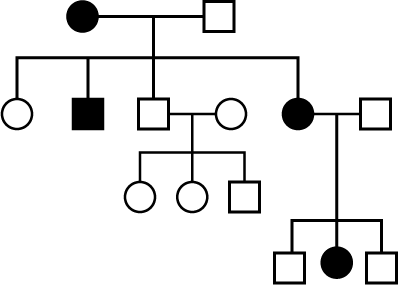 The trait under study is
The trait under study is- a)dominant X-linked
- b)recessive X-linked
- c)autosomal dominant
- d)recessive Y-linked
Correct answer is option 'C'. Can you explain this answer?
Study the pedigree chart of a family showing the inheritance of myotonic dsytrophy

The trait under study is
a)
dominant X-linked
b)
recessive X-linked
c)
autosomal dominant
d)
recessive Y-linked
|
|
Jyoti Sengupta answered |
Autosomal domionant trait can express its effect in homozygpous as well as heterzygous condition.
Given pedigree chart depicts the inheritance of attached ear lobes, an autosomal recessive trait.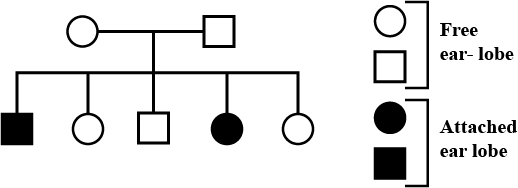 Which of the following conclusions drawn is correct?
Which of the following conclusions drawn is correct?- a)Parents are heterozygous
- b)Parents are homozygous dominant
- c)Parents are homozygous recessive
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Given pedigree chart depicts the inheritance of attached ear lobes, an autosomal recessive trait.

Which of the following conclusions drawn is correct?
a)
Parents are heterozygous
b)
Parents are homozygous dominant
c)
Parents are homozygous recessive
d)
None of these
|
|
Vivek Patel answered |
Given pedigree can be illustrated as follows:
Study the given pedigree chart showing the inheritance of an X-linked trait controlled by gene 'r'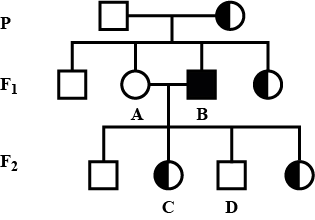 What will be the genotypes of individuals A, B, C and D respectively?
What will be the genotypes of individuals A, B, C and D respectively?- a)XX , XrY, XrX, XY
- b)XrXr , XY, XX, XY
- c)XrX, XrYr, XrXr, XrY
- d)XX, XrYr, XX, XY
Correct answer is option 'A'. Can you explain this answer?
Study the given pedigree chart showing the inheritance of an X-linked trait controlled by gene 'r'

What will be the genotypes of individuals A, B, C and D respectively?
a)
XX , XrY, XrX, XY
b)
XrXr , XY, XX, XY
c)
XrX, XrYr, XrXr, XrY
d)
XX, XrYr, XX, XY
|
|
Anjali Sharma answered |
Genotypes of different individuals in the given pedigree chart can be illustrated as:
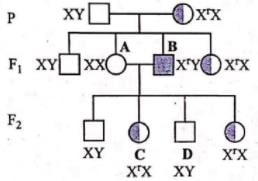
For the following SN reaction, 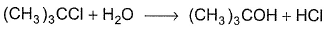 Rate-determining step is
Rate-determining step is- a)
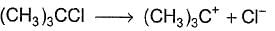
- b)
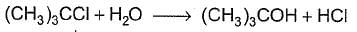
- c)
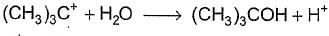
- d)

Correct answer is option 'A'. Can you explain this answer?
For the following SN reaction, 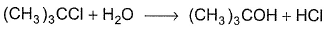
Rate-determining step is
a)
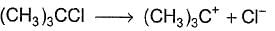
b)
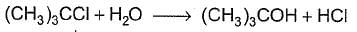
c)
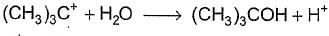
d)


|
Aravind Mehra answered |
H2O is a weak nucleophile and cannot attack tertiary carbon of maximum electron density directly. Thus, rate-determining slow step is
step is
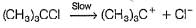
and rate law is
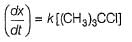
and [H2O] is not involved.
It is SN1 reaction.
Passage IIN2O5 decomposes according to the following equation: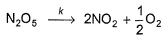 The following mechanism has been given:
The following mechanism has been given: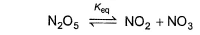
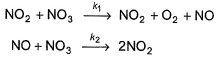 Q.Rate of formation of O2 is given by
Q.Rate of formation of O2 is given by- a)
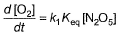
- b)
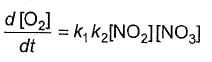
- c)
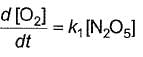
- d)

Correct answer is option 'A'. Can you explain this answer?
Passage II
N2O5 decomposes according to the following equation:
The following mechanism has been given:
Q.
Rate of formation of O2 is given by
a)
b)
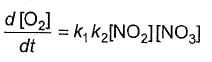
c)
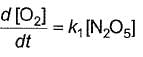
d)


|
Bhargavi Banerjee answered |
Half-life period is independent of [N2O5]. Hence, it is first order reaction.
The following reaction obey rate law 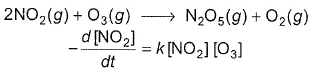 It can follow mechanismI. NO2 + O3
It can follow mechanismI. NO2 + O3 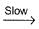 NO3 + O2 , NO3+NO2
NO3 + O2 , NO3+NO2 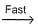 N2O5II. NO2 + O
N2O5II. NO2 + O 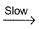 NO3NO3+NO2
NO3NO3+NO2 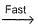 N2O5Select the correct mechanism.
N2O5Select the correct mechanism.- a)Only I
- b)Only II
- c)Both I and II
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
The following reaction obey rate law
It can follow mechanism
I. NO2 + O3 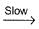 NO3 + O2 ,
NO3 + O2 ,
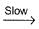 NO3 + O2 ,
NO3 + O2 , NO3+NO2 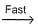 N2O5
N2O5
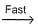 N2O5
N2O5II. NO2 + O  NO3
NO3
 NO3
NO3NO3+NO2  N2O5
N2O5
 N2O5
N2O5Select the correct mechanism.
a)
Only I
b)
Only II
c)
Both I and II
d)
None of these

|
Preethi Bose answered |
In I, slow step is
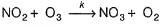
Thus,
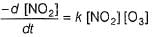
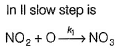
∴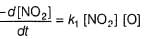
In fast reversible step,
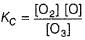
∴
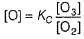
∴
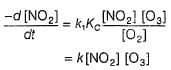
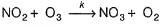
Thus,
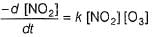
∴
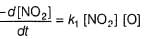
In fast reversible step,
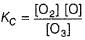
∴
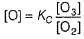
∴
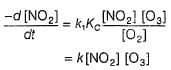
Consider the three following proposed mechanism for the overall equation.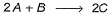
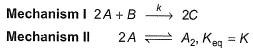
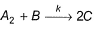
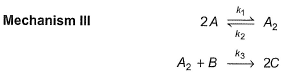 Q.Which mechanism predicts a third order reaction?
Q.Which mechanism predicts a third order reaction? - a)Only I
- b)I and II
- c)II and III
- d)I, II and III
Correct answer is option 'D'. Can you explain this answer?
Consider the three following proposed mechanism for the overall equation.
Q.
Which mechanism predicts a third order reaction?
a)
Only I
b)
I and II
c)
II and III
d)
I, II and III

|
Aarya Dasgupta answered |
It is second order in A and first order in B thus, total order = 3.
II.
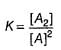
∴
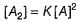
Thus, it is second order in A and first order in B.
Total order = 3
Thus, it is second order in A and first order in B.
Thus total order = 3
Chapter doubts & questions for July Week 3 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of July Week 3 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup