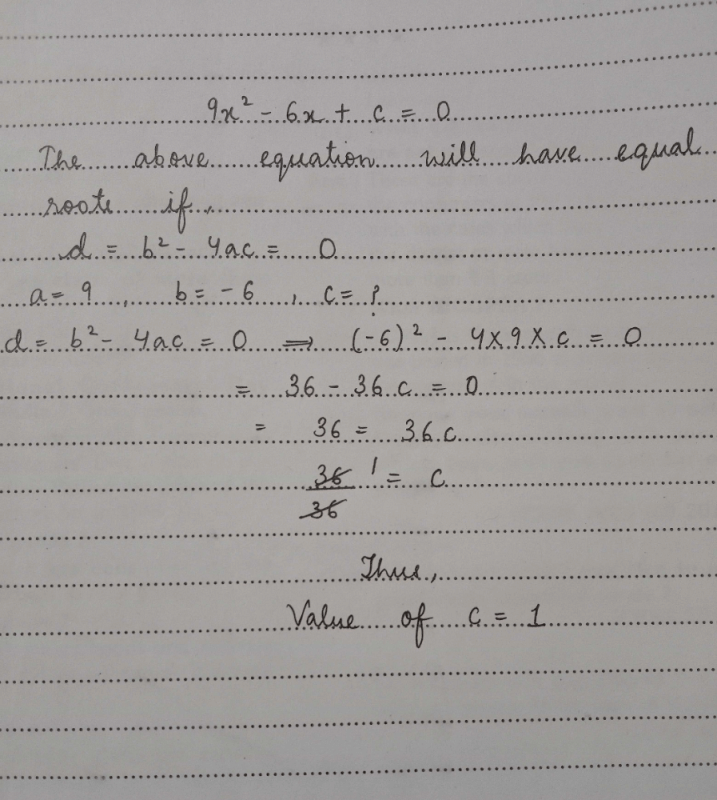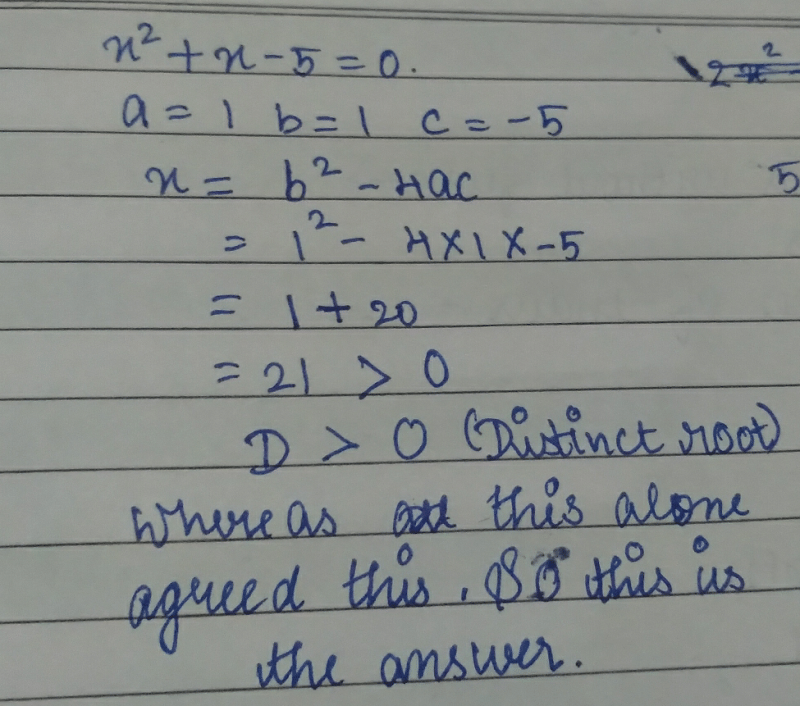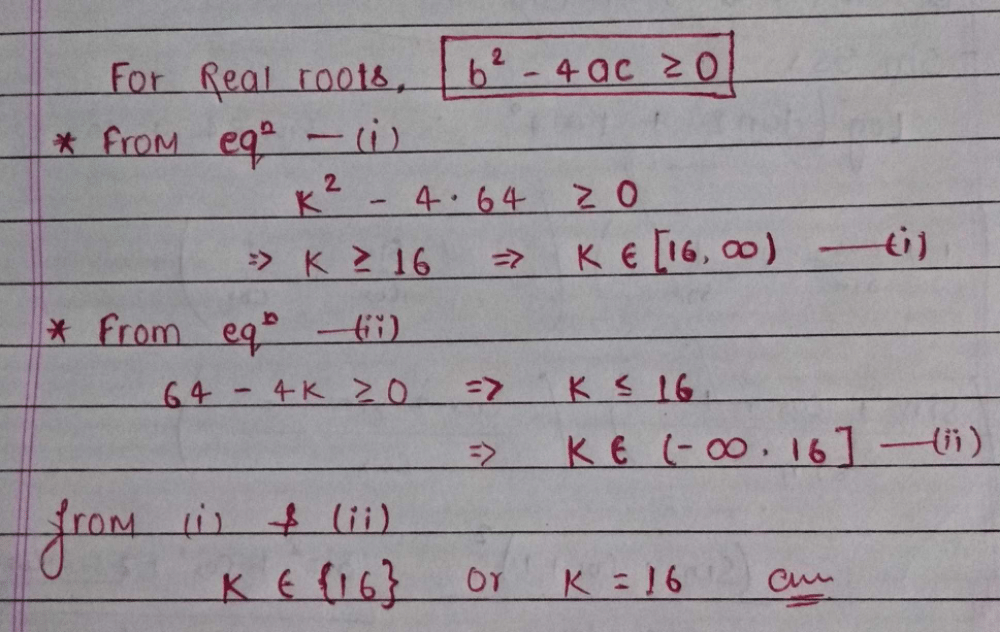All Exams >
Class 10 >
Weekly Tests for Class 10 Preparation >
All Questions
All questions of May Week 4 for Class 10 Exam
Which metal is present in Calcium Hydroxide?- a)C
- b)O
- c)Ca
- d)H
Correct answer is option 'C'. Can you explain this answer?
Which metal is present in Calcium Hydroxide?
a)
C
b)
O
c)
Ca
d)
H
|
|
Amit Sharma answered |
Calcium hydroxide Ca ( OH) 2 , has calcium ( Ca) which is a metal.
Which of the following non-metal is good conductor of electricity?- a)Graphite
- b)Phosphorus
- c)Hydrogen
- d)Bromine
Correct answer is option 'A'. Can you explain this answer?
Which of the following non-metal is good conductor of electricity?
a)
Graphite
b)
Phosphorus
c)
Hydrogen
d)
Bromine
|
|
Gaurav Kumar answered |
Carbon, in the form of Graphite is a good conductor of electricity. It conducts heat and electricity like a metal or a metalloid.
Which one of the following metal reacts vigorously with oxygen and water?
- a)Sodium
- b)Iron
- c)Calcium
- d)Magnesium
Correct answer is option 'A'. Can you explain this answer?
Which one of the following metal reacts vigorously with oxygen and water?
a)
Sodium
b)
Iron
c)
Calcium
d)
Magnesium
|
|
Ananya Das answered |
Sodium metal reacts vigorously with oxygen and water.
MCQ (Multiple Choice Questions) or Practice Quiz with solutions of Chapter - "Metals and Non-Metals" of Class 10 Science, the questions are available for practice Q. By which reaction metal is obtained from metal oxide ?- a)Liquefaction
- b)Reduction
- c)Calcination
- d)Roasting
Correct answer is option 'B'. Can you explain this answer?
MCQ (Multiple Choice Questions) or Practice Quiz with solutions of Chapter - "Metals and Non-Metals" of Class 10 Science, the questions are available for practice
Q. By which reaction metal is obtained from metal oxide ?
a)
Liquefaction
b)
Reduction
c)
Calcination
d)
Roasting
|
|
Vikas Kumar answered |
The method used to extract metals from the ore in which they are found depends on their reactivity. For example, reactive metals such as aluminium are extracted by electrolysis, while a less-reactive metal such as iron may be extracted by reduction with carbon or carbon monoxide.
Which of the following is not a quadratic equation ?- a)5x + 3y2 = 0
- b)z2 - 2z = 0
- c)3x + 4 - 7x2 = 0
- d)5x2 - 125 = 0
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not a quadratic equation ?
a)
5x + 3y2 = 0
b)
z2 - 2z = 0
c)
3x + 4 - 7x2 = 0
d)
5x2 - 125 = 0
|
|
Priyanshu Intelligent answered |
In equation first there are two variables.
That's why it is not a quadratic equation.
That's why it is not a quadratic equation.
The property of metals by which they can be beaten in to thin sheets is called-- a)Malleability
- b)Ductility
- c)Conduction
- d)Expansion
Correct answer is option 'A'. Can you explain this answer?
The property of metals by which they can be beaten in to thin sheets is called-
a)
Malleability
b)
Ductility
c)
Conduction
d)
Expansion
|
|
Ishan Choudhury answered |
Malleability: The property of metals by which they can be beaten into thin sheets is known as malleability.
For example, silver metal is beaten to make silver foil used for decorating sweets.
For example, silver metal is beaten to make silver foil used for decorating sweets.
Metal A when dipped in solution of salt of metal B ,then metal B is displaced . this shows that- a)Metal A is more reactive than metal B
- b)Metal B is more reactive than metal A
- c)Metal A and metal B are equally reactive
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
Metal A when dipped in solution of salt of metal B ,then metal B is displaced . this shows that
a)
Metal A is more reactive than metal B
b)
Metal B is more reactive than metal A
c)
Metal A and metal B are equally reactive
d)
None of these
|
|
Kiran Mehta answered |
When metal A is dipped in a solution of salt of metal V, then metal B is displaced, this shows that metal A is more reactive than B. Only then it can displace B from its salt solution.
A non-metallic oxide which is neutral in nature is- a)CO
- b)CO2
- c)P2O5
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
A non-metallic oxide which is neutral in nature is
a)
CO
b)
CO2
c)
P2O5
d)
None of these
|
|
Amit Sharma answered |
Non - metals generally form acidic oxides but oxides of carbon and nitrogen are neutral in nature like CO and NO.
Which of the following metals will not react with oxygen,even when heated very strongly in air?- a)Zn
- b)Al
- c)Ag
- d)Fe
Correct answer is option 'C'. Can you explain this answer?
Which of the following metals will not react with oxygen,even when heated very strongly in air?
a)
Zn
b)
Al
c)
Ag
d)
Fe
|
|
Ananya Das answered |
Silver do not react with oxygen even at high temperatures as they are less reactive and are placed below the reactivity series. Hence they are called noble metals. But silver fades after some months or years due to its tendency to react with sulphur in air forming their sulphides
If one root of a Quadratic equation is m + , then the other root is- a)m – √n
- b)m +√n
- c)Can not be determined
- d)√m + n
Correct answer is option 'A'. Can you explain this answer?
If one root of a Quadratic equation is m + , then the other root is
a)
m – √n
b)
m +√n
c)
Can not be determined
d)
√m + n
|
|
Arun Sharma answered |
In a quadratic equation with rational coefficients has an irrational root α + √β, then it has a conjugate root α - √β.
So if the root is m+ √n the other root will be m- √n
So if the root is m+ √n the other root will be m- √n
The roots of x2 – 8x + 12 = 0, are- a)x = 0
- b)no real roots
- c)real and unequal
- d)real and equal
Correct answer is option 'C'. Can you explain this answer?
The roots of x2 – 8x + 12 = 0, are
a)
x = 0
b)
no real roots
c)
real and unequal
d)
real and equal
|
|
Genius answered |
9th standard me kya kr rhe the ?!
If a,b,c are real and b2-4ac >0 then roots of equation are- a)real roots
- b)real and equal
- c)real and unequal
- d)No real roots
Correct answer is option 'C'. Can you explain this answer?
If a,b,c are real and b2-4ac >0 then roots of equation are
a)
real roots
b)
real and equal
c)
real and unequal
d)
No real roots
|
|
Ram trivedi answered |
The expression b^2 - 4ac is the discriminant of a quadratic equation of the form ax^2 + bx + c = 0. It determines the nature of the solutions of the equation.
If b^2 - 4ac > 0, then the quadratic equation has two distinct real solutions.
If b^2 - 4ac = 0, then the quadratic equation has one real solution (also known as a double root).
If b^2 - 4ac < 0,="" then="" the="" quadratic="" equation="" has="" no="" real="" solutions.="" however,="" it="" may="" have="" two="" complex="" />
So, in summary, if b^2 - 4ac > 0, there are two real solutions.
If b^2 - 4ac > 0, then the quadratic equation has two distinct real solutions.
If b^2 - 4ac = 0, then the quadratic equation has one real solution (also known as a double root).
If b^2 - 4ac < 0,="" then="" the="" quadratic="" equation="" has="" no="" real="" solutions.="" however,="" it="" may="" have="" two="" complex="" />
So, in summary, if b^2 - 4ac > 0, there are two real solutions.
If -3 is a root of both the Quadratic equations 2x2 + px – 15 = 0, and p(x2 + x) + q = 0, then the value of q if the equation containing it has equal roots is- a)1/2
- b)1/4
- c)14
- d)2
Correct answer is option 'B'. Can you explain this answer?
If -3 is a root of both the Quadratic equations 2x2 + px – 15 = 0, and p(x2 + x) + q = 0, then the value of q if the equation containing it has equal roots is
a)
1/2
b)
1/4
c)
14
d)
2
|
|
Ananya Das answered |
-3 is root of both
2(9) -3p -15 = 0
p = 1
and
second equation has two equal roots so,
b2-4ac = 0
p2 = 4pq
p = 4q
q = 1/4
Which is the least conductor of heat –- a)Gold
- b)Platinum
- c)Silver
- d)Lead
Correct answer is option 'D'. Can you explain this answer?
Which is the least conductor of heat –
a)
Gold
b)
Platinum
c)
Silver
d)
Lead
|
|
Krishna Iyer answered |
Lead being metal is a bad conductor of electricity and heat. Compared to other metals, lead is a poor conductor of heat while gold, silver, platinum are good conductors of heat.
Who was a jobber?- a)A person employed by the industrialist to get new recruits
- b)A person employed by the farmers to sell their products
- c)A person, who was doing the most important job in a factory
- d)A paid servant of the East India Company
Correct answer is option 'A'. Can you explain this answer?
Who was a jobber?
a)
A person employed by the industrialist to get new recruits
b)
A person employed by the farmers to sell their products
c)
A person, who was doing the most important job in a factory
d)
A paid servant of the East India Company
|
|
Evil Sharman answered |
Correct answer is option A !!
: ‘Jobbers’ were usually employed by the industrialists to recruit the right people for work from villages out of the various job seekers
: ‘Jobbers’ were usually employed by the industrialists to recruit the right people for work from villages out of the various job seekers
From which of the following trade did the early entrepreneurs make a fortune?- a)Textile trade
- b)China trade
- c)Trade in tea
- d)Industries
Correct answer is option 'B'. Can you explain this answer?
From which of the following trade did the early entrepreneurs make a fortune?
a)
Textile trade
b)
China trade
c)
Trade in tea
d)
Industries
|
|
Pallavi kapoor answered |
Early Entrepreneurs and China Trade
- Early entrepreneurs made a fortune from China trade.
- China was an important trading partner for many countries including Britain and America.
- The China trade refers to the trade between China and the rest of the world, which was mainly focused on tea, silk, porcelain, and spices.
- The early entrepreneurs were involved in trading these goods between China and the western countries.
- They established trading companies, such as the British East India Company and the Dutch East India Company, to facilitate the trade with China and other Asian countries.
- These companies had a monopoly on the trade with China and were able to make huge profits from the sale of tea, silk, and other goods in Europe and America.
- The early entrepreneurs also played a key role in introducing Chinese tea and porcelain to the western world, which became very popular and valuable commodities.
- The China trade was a major contributor to the growth of the global economy and the development of international trade.
- The early entrepreneurs who were involved in the China trade became very wealthy and influential, and their success inspired others to follow in their footsteps and explore new opportunities for trade and commerce.
- Early entrepreneurs made a fortune from China trade.
- China was an important trading partner for many countries including Britain and America.
- The China trade refers to the trade between China and the rest of the world, which was mainly focused on tea, silk, porcelain, and spices.
- The early entrepreneurs were involved in trading these goods between China and the western countries.
- They established trading companies, such as the British East India Company and the Dutch East India Company, to facilitate the trade with China and other Asian countries.
- These companies had a monopoly on the trade with China and were able to make huge profits from the sale of tea, silk, and other goods in Europe and America.
- The early entrepreneurs also played a key role in introducing Chinese tea and porcelain to the western world, which became very popular and valuable commodities.
- The China trade was a major contributor to the growth of the global economy and the development of international trade.
- The early entrepreneurs who were involved in the China trade became very wealthy and influential, and their success inspired others to follow in their footsteps and explore new opportunities for trade and commerce.
Who was a ‘Jobber’?- a)Trusted worker
- b)Painter
- c)Dancer
- d)Soldier
Correct answer is option 'A'. Can you explain this answer?
Who was a ‘Jobber’?
a)
Trusted worker
b)
Painter
c)
Dancer
d)
Soldier
|
|
Harsh Sharma answered |
I'm sorry, your sentence is incomplete. Could you please provide more information or context?
A real number is said to be a root of ax2+bx+c = 0- a)
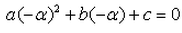
- b)
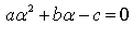
- c)
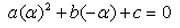
- d)
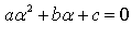
Correct answer is option 'D'. Can you explain this answer?
A real number is said to be a root of ax2+bx+c = 0
a)
b)
c)
d)
|
|
Sameer Diwan answered |
Ni pta re bhai kya likha hai kuch samaj bhi ni aa ra
The most abundant metal in the earth crust is – - a)Al
- b)Fe
- c)O
- d)Cu
Correct answer is option 'A'. Can you explain this answer?
The most abundant metal in the earth crust is –
a)
Al
b)
Fe
c)
O
d)
Cu
|
|
Krishna Iyer answered |
The most abundant Element on the earth's crust is oxygen followed by silicon.. But both of them are Non-metals. But, silicon is followed by Aluminium which is a METAL (about 8.23%) further followed by iron. So the most abundant metal found on the Earth is Aluminium.
The non-metal which is a liquid at room temperature–- a)Oxygen
- b)Fluorine
- c)Sulphur
- d)Bromine
Correct answer is option 'D'. Can you explain this answer?
The non-metal which is a liquid at room temperature–
a)
Oxygen
b)
Fluorine
c)
Sulphur
d)
Bromine
|
|
Abhishek Phogat answered |
Bromine is liquid at room temperature...
The property of metal by which it can be drawn into wires is called- a)Conductivity
- b)Malleability
- c)Ductility
- d)Decorating
Correct answer is option 'C'. Can you explain this answer?
The property of metal by which it can be drawn into wires is called
a)
Conductivity
b)
Malleability
c)
Ductility
d)
Decorating
|
|
Nishu Deshwal answered |
Ductility is correct. Ex- copper, it's used in electric wires because of it's property of ductility and conductivity.
An iron nail is dipped in copper sulphate solution. It is observed that- a)The colour of the solution remain unchanged
- b)The colour of the solution becomes red .
- c)The colour of the solution turns to light green
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
An iron nail is dipped in copper sulphate solution. It is observed that
a)
The colour of the solution remain unchanged
b)
The colour of the solution becomes red .
c)
The colour of the solution turns to light green
d)
None of these
|
|
Arun Sharma answered |
When an iron nail immersed in the solution of copper sulphate than iron displaces copper from the solution of copper sulphate because iron is more reactive than copper. Therefore, copper sulphate solution colour changes from blue to light green .
Which of the following metals is extracted only by electrolysis?- a)Zn
- b)Al
- c)Fe
- d)Cu
Correct answer is option 'B'. Can you explain this answer?
Which of the following metals is extracted only by electrolysis?
a)
Zn
b)
Al
c)
Fe
d)
Cu
|
|
Anmol tiwari answered |
Electrolysis and Metal Extraction
Electrolysis is a process that uses an electric current to drive a non-spontaneous chemical reaction. It is commonly used for metal extraction from their ores. During electrolysis, the metal ions present in the compound are reduced at the cathode (negative electrode), while oxidation occurs at the anode (positive electrode).
Aluminium Extraction
Aluminium is extracted through the process of electrolysis. It is obtained from its ore, bauxite, which is primarily composed of aluminium oxide (Al2O3). The extraction of aluminium involves the following steps:
1. Extraction of Alumina: Bauxite ore is first purified to obtain alumina (Al2O3) through the Bayer's process. In this process, bauxite is dissolved in sodium hydroxide (NaOH) solution, and impurities are removed. The resulting solution is then cooled and filtered to obtain pure alumina.
2. Electrolysis of Alumina: The alumina obtained in the previous step is mixed with cryolite (Na3AlF6) and heated to form a molten electrolyte. This mixture reduces the melting point of alumina, making the electrolysis process more efficient. The molten electrolyte is then electrolyzed using carbon electrodes.
At the cathode: Aluminium ions (Al3+) are reduced and deposited as molten aluminium metal (Al).
At the anode: Oxygen ions (O2-) are oxidized to form oxygen gas (O2).
Since aluminium extraction involves the use of electrolysis, it is the correct answer for the given question.
Other Metals Extraction
- Zinc (Zn): Zinc is not extracted by electrolysis. It is primarily obtained from its ore, zinc blende (ZnS), through a process called roasting and smelting.
- Iron (Fe): Iron is extracted from its ore, hematite (Fe2O3), through a process called reduction with carbon. Electrolysis is not involved in the extraction of iron.
- Copper (Cu): Copper is primarily obtained from its ore, copper pyrite (CuFeS2), through a process called smelting. Electrolysis is not used in the extraction of copper.
Therefore, among the given options, aluminium (Al) is the only metal that is extracted using electrolysis.
Electrolysis is a process that uses an electric current to drive a non-spontaneous chemical reaction. It is commonly used for metal extraction from their ores. During electrolysis, the metal ions present in the compound are reduced at the cathode (negative electrode), while oxidation occurs at the anode (positive electrode).
Aluminium Extraction
Aluminium is extracted through the process of electrolysis. It is obtained from its ore, bauxite, which is primarily composed of aluminium oxide (Al2O3). The extraction of aluminium involves the following steps:
1. Extraction of Alumina: Bauxite ore is first purified to obtain alumina (Al2O3) through the Bayer's process. In this process, bauxite is dissolved in sodium hydroxide (NaOH) solution, and impurities are removed. The resulting solution is then cooled and filtered to obtain pure alumina.
2. Electrolysis of Alumina: The alumina obtained in the previous step is mixed with cryolite (Na3AlF6) and heated to form a molten electrolyte. This mixture reduces the melting point of alumina, making the electrolysis process more efficient. The molten electrolyte is then electrolyzed using carbon electrodes.
At the cathode: Aluminium ions (Al3+) are reduced and deposited as molten aluminium metal (Al).
At the anode: Oxygen ions (O2-) are oxidized to form oxygen gas (O2).
Since aluminium extraction involves the use of electrolysis, it is the correct answer for the given question.
Other Metals Extraction
- Zinc (Zn): Zinc is not extracted by electrolysis. It is primarily obtained from its ore, zinc blende (ZnS), through a process called roasting and smelting.
- Iron (Fe): Iron is extracted from its ore, hematite (Fe2O3), through a process called reduction with carbon. Electrolysis is not involved in the extraction of iron.
- Copper (Cu): Copper is primarily obtained from its ore, copper pyrite (CuFeS2), through a process called smelting. Electrolysis is not used in the extraction of copper.
Therefore, among the given options, aluminium (Al) is the only metal that is extracted using electrolysis.
The roots of the equation x2 – 3x – m (m + 3) = 0, where m is a constant, are- a)–m, m + 3
- b)m, m + 3
- c)–m, – (m + 3)
- d)m, – (m+3)
Correct answer is option 'A'. Can you explain this answer?
The roots of the equation x2 – 3x – m (m + 3) = 0, where m is a constant, are
a)
–m, m + 3
b)
m, m + 3
c)
–m, – (m + 3)
d)
m, – (m+3)
|
|
Aditya Shah answered |
X^2 - 3x - m(m+3) = 0
=> x^2 + mx - (m+3)x- m(m+3) = 0
=> x(x+m) - (m+3)(x+m) = 0
=> (x+m) (x-m-3) = 0
x = - m and m+3
Roots are - m and m+3
The real roots of a quadratic equation 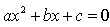 are given by
are given by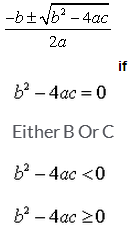
- a)A
- b)B
- c)C
- d)D
Correct answer is 'D'. Can you explain this answer?
The real roots of a quadratic equation 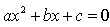 are given by
are given by
a)
A
b)
B
c)
C
d)
D
|
|
Ishan Choudhury answered |
When b2 - 4ac=0 then we have the root as -b/2a.
When b2-4ac < 0,then the root is a complex root ,since we have a negative number ,for which real square root is not possible.
When b2 - 4ac > 0 then we have a square root of it and hence we have real roots. So the correct answer is b2 - 4ac > 0
When b2-4ac < 0,then the root is a complex root ,since we have a negative number ,for which real square root is not possible.
When b2 - 4ac > 0 then we have a square root of it and hence we have real roots. So the correct answer is b2 - 4ac > 0
The sum of the ages of two friends is 20 years. Four years ago, the product of their ages in years was 48. Find their present ages- a)2 and 18
- b)The situation is not possible
- c)6 and 14
- d)10 and 10
Correct answer is option 'B'. Can you explain this answer?
The sum of the ages of two friends is 20 years. Four years ago, the product of their ages in years was 48. Find their present ages
a)
2 and 18
b)
The situation is not possible
c)
6 and 14
d)
10 and 10
|
|
Ayush Iyer answered |
Let's assume the present ages of the two friends are x and y.
According to the given information, the sum of their ages is 20 years.
So, we can write the equation: x + y = 20.
Four years ago, the product of their ages was 48.
So, four years ago, their ages would have been x - 4 and y - 4.
The product of their ages four years ago is: (x - 4)(y - 4) = 48.
Now, let's solve these two equations to find the values of x and y.
Solving the first equation, x + y = 20, we can express x in terms of y:
x = 20 - y.
Substituting the value of x in the second equation, we have:
(20 - y - 4)(y - 4) = 48.
Simplifying this equation, we get:
(16 - y)(y - 4) = 48
16y - 4y - 64 = 48
12y = 112
y = 9.33.
Since y is not a whole number, it means there are no two whole numbers that satisfy the given conditions. Therefore, the situation is not possible.
Hence, the correct answer is option B - The situation is not possible.
According to the given information, the sum of their ages is 20 years.
So, we can write the equation: x + y = 20.
Four years ago, the product of their ages was 48.
So, four years ago, their ages would have been x - 4 and y - 4.
The product of their ages four years ago is: (x - 4)(y - 4) = 48.
Now, let's solve these two equations to find the values of x and y.
Solving the first equation, x + y = 20, we can express x in terms of y:
x = 20 - y.
Substituting the value of x in the second equation, we have:
(20 - y - 4)(y - 4) = 48.
Simplifying this equation, we get:
(16 - y)(y - 4) = 48
16y - 4y - 64 = 48
12y = 112
y = 9.33.
Since y is not a whole number, it means there are no two whole numbers that satisfy the given conditions. Therefore, the situation is not possible.
Hence, the correct answer is option B - The situation is not possible.
Which of the following metal can we cut with the knife –- a)Gold
- b)Potassium
- c)Iron
- d)All of these
Correct answer is option 'B'. Can you explain this answer?
Which of the following metal can we cut with the knife –
a)
Gold
b)
Potassium
c)
Iron
d)
All of these
|
|
Pooja Shah answered |
There is lot of empty space on the unit cell, hence metallic bond is weaker. Thus sodium and potassium appear soft and can with knife.
Which non-metallic element is in liquid form ? - a)Carbon
- b)Hydrogen
- c)Bromine
- d)Phosphorus
Correct answer is option 'C'. Can you explain this answer?
Which non-metallic element is in liquid form ?
a)
Carbon
b)
Hydrogen
c)
Bromine
d)
Phosphorus
|
|
Krishna Iyer answered |
The metal which exist as a liquid at room temperature is mercury. Non - metal which exist as a liquid at room temperature is bromine.
Why did the weavers suffer from a problem of raw cotton?- a)The cotton crop perished
- b)Raw cotton exports increased
- c)Local markets shrank
- d)Export market collapsed
Correct answer is option 'B'. Can you explain this answer?
Why did the weavers suffer from a problem of raw cotton?
a)
The cotton crop perished
b)
Raw cotton exports increased
c)
Local markets shrank
d)
Export market collapsed
|
|
Shambavi menon answered |
The weavers suffered from a problem of raw cotton because of the following reason:
Increase in Raw Cotton Exports
- British colonial policy encouraged the cultivation of raw cotton in India for export to Britain's textile mills.
- This led to an increase in the export of raw cotton, which resulted in a shortage of raw cotton for local weavers.
Impact on Weavers
- With a shortage of raw cotton, weavers had to pay higher prices for the raw material they needed to produce their textiles.
- This made it difficult for them to compete with the cheaper textiles produced in Britain's mills.
Loss of Livelihoods
- As the demand for locally produced textiles declined, many weavers lost their livelihoods.
- This led to widespread poverty and forced many weavers to migrate to other areas in search of work.
Conclusion
- The problem of raw cotton was a major factor in the decline of the handloom industry in India during the colonial period.
- It highlights how colonial policies and economic structures can have a profound impact on local industries and livelihoods.
Increase in Raw Cotton Exports
- British colonial policy encouraged the cultivation of raw cotton in India for export to Britain's textile mills.
- This led to an increase in the export of raw cotton, which resulted in a shortage of raw cotton for local weavers.
Impact on Weavers
- With a shortage of raw cotton, weavers had to pay higher prices for the raw material they needed to produce their textiles.
- This made it difficult for them to compete with the cheaper textiles produced in Britain's mills.
Loss of Livelihoods
- As the demand for locally produced textiles declined, many weavers lost their livelihoods.
- This led to widespread poverty and forced many weavers to migrate to other areas in search of work.
Conclusion
- The problem of raw cotton was a major factor in the decline of the handloom industry in India during the colonial period.
- It highlights how colonial policies and economic structures can have a profound impact on local industries and livelihoods.
If the value of the Discriminant function of a quadratic equation is D = 27, then its roots are- a)Distinct, Rational
- b)Same Irrational
- c)Distinct, Irrational
- d) Same, Rational
Correct answer is option 'C'. Can you explain this answer?
If the value of the Discriminant function of a quadratic equation is D = 27, then its roots are
a)
Distinct, Rational
b)
Same Irrational
c)
Distinct, Irrational
d)
Same, Rational
|
|
Roshni chauhan answered |
Quadratic equation is a polynomial equation of degree two, which can be written in the form of ax² + bx + c = 0. The discriminant of a quadratic equation is given by D = b² - 4ac. It is a function of the coefficients of the quadratic equation and is used to determine the nature of the roots of the equation.
Distinct and Irrational Roots
If the value of the discriminant is positive and a perfect square, then the roots of the quadratic equation are distinct and rational. If the value of the discriminant is positive but not a perfect square, then the roots of the quadratic equation are distinct and irrational.
Same and Rational Roots
If the value of the discriminant is zero, then the roots of the quadratic equation are same and rational. If the value of the discriminant is negative, then the roots of the quadratic equation are complex conjugates.
Given, D = 27
From the above discussion, we know that if the value of the discriminant is positive and not a perfect square, then the roots of the quadratic equation are distinct and irrational.
Therefore, the correct answer is option C, which states that the roots of the quadratic equation are distinct and irrational.
Distinct and Irrational Roots
If the value of the discriminant is positive and a perfect square, then the roots of the quadratic equation are distinct and rational. If the value of the discriminant is positive but not a perfect square, then the roots of the quadratic equation are distinct and irrational.
Same and Rational Roots
If the value of the discriminant is zero, then the roots of the quadratic equation are same and rational. If the value of the discriminant is negative, then the roots of the quadratic equation are complex conjugates.
Given, D = 27
From the above discussion, we know that if the value of the discriminant is positive and not a perfect square, then the roots of the quadratic equation are distinct and irrational.
Therefore, the correct answer is option C, which states that the roots of the quadratic equation are distinct and irrational.
Which of the following is a solution of the quadratic equation x2 - b2 = a (2x - a)- a)x = a/b
- b)x = ab
- c)x = a+b
- d)x = b/a
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a solution of the quadratic equation x2 - b2 = a (2x - a)
a)
x = a/b
b)
x = ab
c)
x = a+b
d)
x = b/a
|
|
Ram Mohith answered |
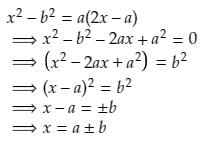
So, x = a + b is one of the solution for the given quadratic equation.
The most abundant metal in the earth crust is – - a)Al
- b)Fe
- c)O
- d)Cu
Correct answer is option 'A'. Can you explain this answer?
The most abundant metal in the earth crust is –
a)
Al
b)
Fe
c)
O
d)
Cu

|
Aisha Negi answered |
Aluminium is the most abundant metal means Found in a larger quantity in the earth crust
Find the incorrect option :
Proto industrial system was.........- a)A part of a network of Commercial exchanges
- b)Controlled by merchants
- c)The system under which goods were produced in factories
- d)A system which supplemented the income of people in the countryside
Correct answer is option 'C'. Can you explain this answer?
Find the incorrect option :
Proto industrial system was.........
Proto industrial system was.........
a)
A part of a network of Commercial exchanges
b)
Controlled by merchants
c)
The system under which goods were produced in factories
d)
A system which supplemented the income of people in the countryside

|
Subha Mondal answered |
Proto industrial system didn't include production of factories.. It was carried out in homes in the countryside. so option C is not correct.
In Victorian Britain, the upper classes-aristocratic class and bourgeoisie preferred handmade goods because:- a)they were made from imported material
- b)the handmade goods came to symbolize refinement and class
- c)they were better finished
- d)only the upper class could afford the expensive items
Correct answer is option 'B'. Can you explain this answer?
In Victorian Britain, the upper classes-aristocratic class and bourgeoisie preferred handmade goods because:
a)
they were made from imported material
b)
the handmade goods came to symbolize refinement and class
c)
they were better finished
d)
only the upper class could afford the expensive items
|
|
Swati Malkani answered |
Option B is correct
Who devised the Spinning Jenny?- a)Samual Luck
- b)Richard Arkwright
- c)James Hargreaves
- d)James Watt
Correct answer is option 'C'. Can you explain this answer?
Who devised the Spinning Jenny?
a)
Samual Luck
b)
Richard Arkwright
c)
James Hargreaves
d)
James Watt
|
|
Sagar chauhan answered |
The correct answer is option 'C', James Hargreaves.
James Hargreaves, an English weaver and carpenter, devised the Spinning Jenny in the late 1760s. The Spinning Jenny was an important invention in the textile industry and played a significant role in the Industrial Revolution.
Below are the details explaining James Hargreaves' role in devising the Spinning Jenny:
1. Background:
- In the 18th century, the demand for cotton textiles was increasing rapidly, but the existing methods of spinning yarn were time-consuming and inefficient.
- Prior to the Spinning Jenny, spinning yarn was done using a spinning wheel, which required significant manual effort and could only produce one thread at a time.
2. Invention of the Spinning Jenny:
- James Hargreaves, who was both a weaver and a carpenter, observed the limitations of the spinning wheel and sought to find a solution.
- In the late 1760s, he developed a machine that could spin multiple threads at once, which he named the Spinning Jenny.
- The Spinning Jenny was a hand-powered machine consisting of a simple frame with multiple spindles and a single wheel.
- By turning the wheel, the operator could simultaneously spin up to eight threads, significantly increasing the productivity of yarn production.
3. Impact and Significance:
- The Spinning Jenny revolutionized the textile industry by increasing the speed and efficiency of yarn production.
- It allowed for the production of multiple threads simultaneously, reducing the labor required and increasing productivity.
- The invention of the Spinning Jenny was a significant step towards mechanization in the textile industry and played a crucial role in the Industrial Revolution.
- The increased production of yarn provided the necessary raw material for the subsequent development of mechanized weaving machines, further transforming the textile industry.
In conclusion, James Hargreaves, an English weaver and carpenter, devised the Spinning Jenny in the late 1760s. This invention revolutionized the textile industry by enabling the simultaneous spinning of multiple threads and significantly increasing productivity. The Spinning Jenny played a crucial role in the Industrial Revolution and paved the way for further mechanization in the textile industry.
James Hargreaves, an English weaver and carpenter, devised the Spinning Jenny in the late 1760s. The Spinning Jenny was an important invention in the textile industry and played a significant role in the Industrial Revolution.
Below are the details explaining James Hargreaves' role in devising the Spinning Jenny:
1. Background:
- In the 18th century, the demand for cotton textiles was increasing rapidly, but the existing methods of spinning yarn were time-consuming and inefficient.
- Prior to the Spinning Jenny, spinning yarn was done using a spinning wheel, which required significant manual effort and could only produce one thread at a time.
2. Invention of the Spinning Jenny:
- James Hargreaves, who was both a weaver and a carpenter, observed the limitations of the spinning wheel and sought to find a solution.
- In the late 1760s, he developed a machine that could spin multiple threads at once, which he named the Spinning Jenny.
- The Spinning Jenny was a hand-powered machine consisting of a simple frame with multiple spindles and a single wheel.
- By turning the wheel, the operator could simultaneously spin up to eight threads, significantly increasing the productivity of yarn production.
3. Impact and Significance:
- The Spinning Jenny revolutionized the textile industry by increasing the speed and efficiency of yarn production.
- It allowed for the production of multiple threads simultaneously, reducing the labor required and increasing productivity.
- The invention of the Spinning Jenny was a significant step towards mechanization in the textile industry and played a crucial role in the Industrial Revolution.
- The increased production of yarn provided the necessary raw material for the subsequent development of mechanized weaving machines, further transforming the textile industry.
In conclusion, James Hargreaves, an English weaver and carpenter, devised the Spinning Jenny in the late 1760s. This invention revolutionized the textile industry by enabling the simultaneous spinning of multiple threads and significantly increasing productivity. The Spinning Jenny played a crucial role in the Industrial Revolution and paved the way for further mechanization in the textile industry.
If 1/2 is a root of the equation x2+kx – (5/4) = 0, then the other root of the quadratic equation is- a)-2
- b)1/4
- c)-5/2
- d)1/2
Correct answer is option 'C'. Can you explain this answer?
If 1/2 is a root of the equation x2+kx – (5/4) = 0, then the other root of the quadratic equation is
a)
-2
b)
1/4
c)
-5/2
d)
1/2
|
|
Varsha das answered |
I'm sorry, but the equation you provided is incomplete. Could you please provide the complete equation?
For what value(s) of k will the equation kx2-5x+k = 0 have a repeated root?- a)±5/2
- b)5/2
- c)-5/2
- d)3/2
Correct answer is option 'A'. Can you explain this answer?
For what value(s) of k will the equation kx2-5x+k = 0 have a repeated root?
a)
±5/2
b)
5/2
c)
-5/2
d)
3/2
|
|
Stuti answered |
Kx^2-5x+k=0
a=k, b=-5, c=k
b^2-4ac=0
(-5)^2 - 4×k×k= 0
25 - 4k^2 = 0
25/4 = k^2
+√25/4 = k
-
+-5/2 ANS.
a=k, b=-5, c=k
b^2-4ac=0
(-5)^2 - 4×k×k= 0
25 - 4k^2 = 0
25/4 = k^2
+√25/4 = k
-
+-5/2 ANS.
What do you mean by the orient? Which of the following meaning is correct?- a)The countries to the west of the Indian Ocean
- b)The countries to the east of the Mediterranean sea
- c)The countries to the west of the Pacific Ocean
- d)The countries east of the Red Sea
Correct answer is option 'B'. Can you explain this answer?
What do you mean by the orient? Which of the following meaning is correct?
a)
The countries to the west of the Indian Ocean
b)
The countries to the east of the Mediterranean sea
c)
The countries to the west of the Pacific Ocean
d)
The countries east of the Red Sea
|
|
Rajiv Gupta answered |
Correct, option B is the correct meaning of "the Orient." The term "Orient" is often used to refer to the countries and regions to the east of the Mediterranean sea, including the Middle East, North Africa, and parts of Asia. It is traditionally used to refer to the East in contrast to the West, and has been used historically in the context of European colonialism and imperialism.
Aluminium is commonly used for making cooking utensils. What properties of aluminium make it suitable for this purpose?(i) Good thermal conductivity(ii) Good electrical conductivity(iii) Ductility(iv) High melting point- a) (i) and (ii)
- b) (i) and (iii)
- c)(ii) and (iii)
- d)(i) and (iv)
Correct answer is option 'D'. Can you explain this answer?
Aluminium is commonly used for making cooking utensils. What properties of aluminium make it suitable for this purpose?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) High melting point
a)
(i) and (ii)
b)
(i) and (iii)
c)
(ii) and (iii)
d)
(i) and (iv)
|
|
Kritika Menon answered |
Properties of Aluminium for Cooking Utensils
Aluminium is widely favored for cooking utensils due to its unique properties that enhance cooking efficiency and safety. The correct answer highlights the importance of good thermal conductivity and high melting point.
Good Thermal Conductivity
- Aluminium has excellent thermal conductivity, allowing it to heat up quickly and distribute heat evenly across the cooking surface.
- This property ensures that food cooks uniformly, reducing the risk of hot spots that can burn food.
High Melting Point
- Aluminium has a relatively high melting point (around 660°C), making it suitable for high-temperature cooking.
- This property ensures that aluminium utensils can withstand the heat of cooking without deforming or melting, providing durability and longevity.
Other Properties Not Essential for Cooking
- While aluminium's good electrical conductivity and ductility are valuable in other applications, they are not critical for cooking utensils.
- Electrical conductivity is more relevant in electrical applications, while ductility is important for shaping and forming materials rather than for cooking performance.
Conclusion
In summary, aluminium's good thermal conductivity and high melting point make it an ideal material for cooking utensils, ensuring efficient cooking and durability. The properties of electrical conductivity and ductility, while beneficial in other contexts, are not essential for the purpose of cooking. Thus, the correct option is 'D' (i) and (iv).
Aluminium is widely favored for cooking utensils due to its unique properties that enhance cooking efficiency and safety. The correct answer highlights the importance of good thermal conductivity and high melting point.
Good Thermal Conductivity
- Aluminium has excellent thermal conductivity, allowing it to heat up quickly and distribute heat evenly across the cooking surface.
- This property ensures that food cooks uniformly, reducing the risk of hot spots that can burn food.
High Melting Point
- Aluminium has a relatively high melting point (around 660°C), making it suitable for high-temperature cooking.
- This property ensures that aluminium utensils can withstand the heat of cooking without deforming or melting, providing durability and longevity.
Other Properties Not Essential for Cooking
- While aluminium's good electrical conductivity and ductility are valuable in other applications, they are not critical for cooking utensils.
- Electrical conductivity is more relevant in electrical applications, while ductility is important for shaping and forming materials rather than for cooking performance.
Conclusion
In summary, aluminium's good thermal conductivity and high melting point make it an ideal material for cooking utensils, ensuring efficient cooking and durability. The properties of electrical conductivity and ductility, while beneficial in other contexts, are not essential for the purpose of cooking. Thus, the correct option is 'D' (i) and (iv).
Chapter doubts & questions for May Week 4 - Weekly Tests for Class 10 Preparation 2025 is part of Class 10 exam preparation. The chapters have been prepared according to the Class 10 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Class 10 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of May Week 4 - Weekly Tests for Class 10 Preparation in English & Hindi are available as part of Class 10 exam.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup