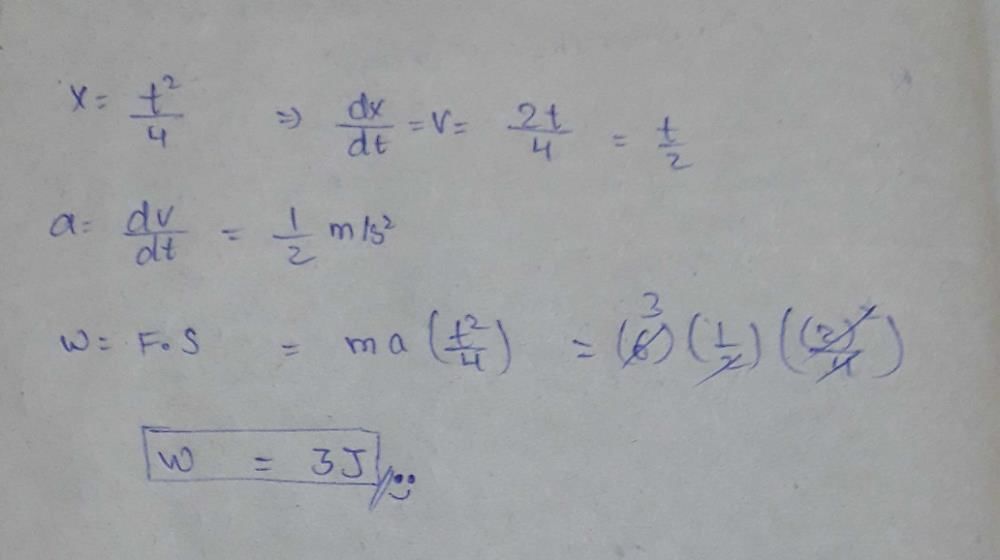All Exams >
JEE >
WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 >
All Questions
All questions of 2011 for JEE Exam
The number of diagonals in a polygon is 20. The number of sides of the polygon is- a)5
- b)6
- c)8
- d)10
Correct answer is option 'C'. Can you explain this answer?
The number of diagonals in a polygon is 20. The number of sides of the polygon is
a)
5
b)
6
c)
8
d)
10
|
|
Anand Kumar answered |
Use formula no.of diagonal = n(n-3)/2
Inner surface of the bronchi, bronchioles and fallopian tubes are lined by- a)cubical epithelium
- b)columnar epithelium
- c)squamous epithelium
- d)ciliated epithelium
Correct answer is option 'D'. Can you explain this answer?
Inner surface of the bronchi, bronchioles and fallopian tubes are lined by
a)
cubical epithelium
b)
columnar epithelium
c)
squamous epithelium
d)
ciliated epithelium
|
|
Kiran Sarmah answered |
Ciliated epithelium lines the inner surface of bronchi, bronchioles and Fallopian tubes. It consists of a single layer of Ciliated rectangular cells .It also lines the oviduct and neurocoel of CNS.
The Gastrin is secreted from- a)Intestine
- b)Stomach
- c)Pancreas
- d)Rectum
Correct answer is option 'B'. Can you explain this answer?
The Gastrin is secreted from
a)
Intestine
b)
Stomach
c)
Pancreas
d)
Rectum
|
|
Khaja Moinuddin answered |
Gastrin is a hormone that is produced by 'G' cells in the lining of the stomach and upper small intestine. During a meal, gastrin stimulates the stomach to release gastric acid.
The coordinates of a moving point p are (2t2 + 4, 4t + 6). Then its locus will be a- a)circle
- b)straight line
- c)parabola
- d)ellipse
Correct answer is option 'C'. Can you explain this answer?
The coordinates of a moving point p are (2t2 + 4, 4t + 6). Then its locus will be a
a)
circle
b)
straight line
c)
parabola
d)
ellipse
|
|
Ashfaque Usmani answered |
Any moving point on any curve is called locus
so given that
h = 2t^2+4
and k=4t+6
t =k-6/4
put the value of t in above equation
h =2 ×(k-6/4)^2 +4
(k-6)^2 = h-4/2
locus of the curve is
(y-6)^2 = x-4/2 so that this equation of parabola
so the correct answer is option C
so given that
h = 2t^2+4
and k=4t+6
t =k-6/4
put the value of t in above equation
h =2 ×(k-6/4)^2 +4
(k-6)^2 = h-4/2
locus of the curve is
(y-6)^2 = x-4/2 so that this equation of parabola
so the correct answer is option C
The normality of 30 volume H2O2 is- a)2.678 N
- b)5.336 N
- c)8.034 N
- d)6.685 N
Correct answer is option 'B'. Can you explain this answer?
The normality of 30 volume H2O2 is
a)
2.678 N
b)
5.336 N
c)
8.034 N
d)
6.685 N
|
|
Rohit Jain answered |
Volume strength = 5.6 × normality
30 = 5.6 × N
N = 30/5.6 = 5.3
The resistance across A and B in the figure below will be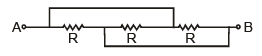
- a)3 R
- b)R
- c)R/3
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
The resistance across A and B in the figure below will be
a)
3 R
b)
R
c)
R/3
d)
None of these
|
|
Lavanya Menon answered |
Resistance are in parallel = R/3
if x + 1/x = 2cosθ, then for any integer n , xn + 1/xn- a)2 cos nθ
- b)2 sin nθ
- c)2i cos nθ
- d)2i sin nθ
Correct answer is option 'A'. Can you explain this answer?
if x + 1/x = 2cosθ, then for any integer n , xn + 1/xn
a)
2 cos nθ
b)
2 sin nθ
c)
2i cos nθ
d)
2i sin nθ
|
|
Yash Goyal answered |
x + 1/x = 2cosθ
Let x = cos θ + 1 sin θ
1/x = cosθ − 1sinθ
thus, xn + 1/xn = 2 cos nθ
Which one of the following human cells do not contain mitochondria ?- a)Nerve cell
- b)Red blood cell
- c)Liver cell
- d)White blood cell
Correct answer is option 'B'. Can you explain this answer?
Which one of the following human cells do not contain mitochondria ?
a)
Nerve cell
b)
Red blood cell
c)
Liver cell
d)
White blood cell
|
|
Ritika Sen answered |
Matured Red blood cells are without mitochondria.
The energy of an electron in first Bohr orbit of H – atom is – 13.6 eV. The possible energy value of electron in the excited state of Li2+ is- a)– 122.4 eV
- b)30.6 eV
- c)– 30.6 eV
- d)13.6 eV
Correct answer is option 'C'. Can you explain this answer?
The energy of an electron in first Bohr orbit of H – atom is – 13.6 eV. The possible energy value of electron in the excited state of Li2+ is
a)
– 122.4 eV
b)
30.6 eV
c)
– 30.6 eV
d)
13.6 eV
|
|
Anirban Desai answered |
For the excited state, n = 2 and for Li++ ion, z = 3
A radioactive atom yxM emits two α particles and one β particle successively. The number of neutrons in the nucleus of the product will be- a)X – 4 – Y
- b)X – Y – 5
- c)X – Y – 3
- d)X – Y – 6
Correct answer is option 'B'. Can you explain this answer?
A radioactive atom yxM emits two α particles and one β particle successively. The number of neutrons in the nucleus of the product will be
a)
X – 4 – Y
b)
X – Y – 5
c)
X – Y – 3
d)
X – Y – 6
|
|
Prasenjit Sen answered |
Alpha particles and one beta particle. After emission, what is the resulting nucleus?
The emission of two alpha particles means that the original nucleus loses two protons and two neutrons. This results in a new nucleus with an atomic number that is two less than the original nucleus.
yxM - 2α = (y-2)(x-4)
The emission of one beta particle means that a neutron in the original nucleus is converted into a proton, while an electron is emitted from the nucleus. This results in a new nucleus with the same atomic number but one more proton.
(y-2)(x-4) - β = (y-2)x
Therefore, the resulting nucleus is (y-2)x with one more proton than the original nucleus.
The emission of two alpha particles means that the original nucleus loses two protons and two neutrons. This results in a new nucleus with an atomic number that is two less than the original nucleus.
yxM - 2α = (y-2)(x-4)
The emission of one beta particle means that a neutron in the original nucleus is converted into a proton, while an electron is emitted from the nucleus. This results in a new nucleus with the same atomic number but one more proton.
(y-2)(x-4) - β = (y-2)x
Therefore, the resulting nucleus is (y-2)x with one more proton than the original nucleus.
If the ratio of the roots of the equation px2 + qx + r = 0 is a : b, then ab/(a + b)2 is- a)p2/qr
- b)pr/q2
- c)q2/pr
- d)pq/r2
Correct answer is option 'B'. Can you explain this answer?
If the ratio of the roots of the equation px2 + qx + r = 0 is a : b, then ab/(a + b)2 is
a)
p2/qr
b)
pr/q2
c)
q2/pr
d)
pq/r2

|
Raja Kotni answered |
Let two roots be 1.is ak,2.is bk where k is constant then find the given condition
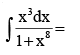
- a)4 tan–1 x3 + c
- b)
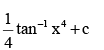
- c)x + 4 tan–1 x4 + c
- d)
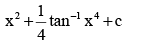
Correct answer is option 'B'. Can you explain this answer?
a)
4 tan–1 x3 + c
b)
c)
x + 4 tan–1 x4 + c
d)

|
Peter Parker answered |
Option B is correct
If g is the acceleration due to gravity on the surface of the earth, the gain in potential energy of an object of mass m raised from the earth’s surface to a height equal to the radius R of the earth is- a)mg R/4
- b)mg R/2
- c)mg R
- d)2mg R
Correct answer is option 'B'. Can you explain this answer?
If g is the acceleration due to gravity on the surface of the earth, the gain in potential energy of an object of mass m raised from the earth’s surface to a height equal to the radius R of the earth is
a)
mg R/4
b)
mg R/2
c)
mg R
d)
2mg R
|
|
Bhavana Gupta answered |
The electronic transitions from n = 2 to n = 1 will produce shortest wavelength in (where n = principal quantum state)- a)Li+2
- b)He+
- c)H
- d)H+
Correct answer is option 'A'. Can you explain this answer?
The electronic transitions from n = 2 to n = 1 will produce shortest wavelength in (where n = principal quantum state)
a)
Li+2
b)
He+
c)
H
d)
H+
|
|
Jay Datta answered |
Explanation:
1. Electronic Transitions:
Electronic transitions occur when an electron in an atom moves from one energy level to another. The energy levels in an atom are represented by principal quantum numbers (n), where n = 1, 2, 3, ... Each energy level corresponds to a specific amount of energy that an electron possesses.
2. Principal Quantum Numbers:
The principal quantum number (n) determines the size and energy of the electron's orbital. The lowest energy level, n = 1, is closest to the nucleus, while higher energy levels are progressively further away. As the distance between the electron and the nucleus increases, the energy of the electron also increases.
3. Shortest Wavelength:
The wavelength of light emitted during an electronic transition is inversely proportional to the energy difference between the initial and final energy levels. The larger the energy difference, the shorter the wavelength of light emitted.
4. Li, He, H:
- Li (Atomic Number 3): Lithium has three electrons. The electronic transition from n = 2 to n = 1 involves the transition of one electron from the second energy level to the first energy level.
- He (Atomic Number 2): Helium has two electrons. The electronic transition from n = 2 to n = 1 involves the transition of one electron from the second energy level to the first energy level.
- H (Atomic Number 1): Hydrogen has one electron. The electronic transition from n = 2 to n = 1 involves the transition of one electron from the second energy level to the first energy level.
5. Reasoning:
The energy difference between the n = 2 and n = 1 energy levels is the greatest for Li compared to He and H. This is because Li has more protons in the nucleus, leading to a stronger attraction between the electron and the nucleus. As a result, the energy required for the electron to transition from the second energy level to the first energy level is higher in Li compared to He and H.
Since the wavelength of light emitted is inversely proportional to the energy difference, Li will produce the shortest wavelength of light among the given options (Li, He, and H) when the electron transitions from the n = 2 to n = 1 energy level.
Therefore, the correct answer is option 'A' (Li).
1. Electronic Transitions:
Electronic transitions occur when an electron in an atom moves from one energy level to another. The energy levels in an atom are represented by principal quantum numbers (n), where n = 1, 2, 3, ... Each energy level corresponds to a specific amount of energy that an electron possesses.
2. Principal Quantum Numbers:
The principal quantum number (n) determines the size and energy of the electron's orbital. The lowest energy level, n = 1, is closest to the nucleus, while higher energy levels are progressively further away. As the distance between the electron and the nucleus increases, the energy of the electron also increases.
3. Shortest Wavelength:
The wavelength of light emitted during an electronic transition is inversely proportional to the energy difference between the initial and final energy levels. The larger the energy difference, the shorter the wavelength of light emitted.
4. Li, He, H:
- Li (Atomic Number 3): Lithium has three electrons. The electronic transition from n = 2 to n = 1 involves the transition of one electron from the second energy level to the first energy level.
- He (Atomic Number 2): Helium has two electrons. The electronic transition from n = 2 to n = 1 involves the transition of one electron from the second energy level to the first energy level.
- H (Atomic Number 1): Hydrogen has one electron. The electronic transition from n = 2 to n = 1 involves the transition of one electron from the second energy level to the first energy level.
5. Reasoning:
The energy difference between the n = 2 and n = 1 energy levels is the greatest for Li compared to He and H. This is because Li has more protons in the nucleus, leading to a stronger attraction between the electron and the nucleus. As a result, the energy required for the electron to transition from the second energy level to the first energy level is higher in Li compared to He and H.
Since the wavelength of light emitted is inversely proportional to the energy difference, Li will produce the shortest wavelength of light among the given options (Li, He, and H) when the electron transitions from the n = 2 to n = 1 energy level.
Therefore, the correct answer is option 'A' (Li).
The correct order of decreasing acidity of nitrophenols will be- a)m-Nitrophenol > p-Nitrophenol > o-Nitrophenol
- b)o-Nitrophenol > m- Nitrophenol > p-Nitrophenol
- c)p-Nitrophenol > m- Nitrophenol > o-Nitrophenol
- d)p-Nitrophenol > o-nitrophenol > m-Nitrophenol
Correct answer is option 'D'. Can you explain this answer?
The correct order of decreasing acidity of nitrophenols will be
a)
m-Nitrophenol > p-Nitrophenol > o-Nitrophenol
b)
o-Nitrophenol > m- Nitrophenol > p-Nitrophenol
c)
p-Nitrophenol > m- Nitrophenol > o-Nitrophenol
d)
p-Nitrophenol > o-nitrophenol > m-Nitrophenol
|
|
Nisheeth Charan answered |
In meta nitrophenol no -m least acidic
in ortho and para -m will be there among them ortho nitro have hydrogen bonding which will decrese acidic nature
in ortho and para -m will be there among them ortho nitro have hydrogen bonding which will decrese acidic nature
In 24 hours, total glomerular filtrate formed in human kidney is- a)1.7 litres
- b)7 litres
- c)17 litres
- d)170 litres
Correct answer is option 'D'. Can you explain this answer?
In 24 hours, total glomerular filtrate formed in human kidney is
a)
1.7 litres
b)
7 litres
c)
17 litres
d)
170 litres
|
|
Sumanth Anju answered |
The volume of filtrate formed by both kidneys per minute is termedglomerular filtration rate (GFR). Approximately 20% of your cardiac output is filtered by your kidneys per minute under resting conditions. The work of the kidneys produces about 125 mL/min filtrate in men (range of 90 to 140 mL/min) and 105 mL/min filtrate in women (range of 80 to 125 mL/min). This amount equates to a volume of about 180 L/day in men and 150 L/day in women. However, 99% of this filtrate is returned to the circulation through reabsorption resulting in only about 1–2 liters of urine per day.
Which of the following compounds is not formed in iodoform reaction of acetone- a)CH3COCH2I
- b)ICH2COCH2I
- c)CH3COCHI2
- d)CH3COCI3
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds is not formed in iodoform reaction of acetone
a)
CH3COCH2I
b)
ICH2COCH2I
c)
CH3COCHI2
d)
CH3COCI3
|
|
Siddharth Sengupta answered |
Explanation:
The iodoform reaction is a test used to determine the presence of a methyl ketone or a compound with a methyl ketone functional group. In this reaction, the methyl ketone reacts with iodine and a base to form a yellow precipitate of iodoform.
The reaction mechanism involves the following steps:
1. The base (usually sodium hydroxide, NaOH) deprotonates the methyl ketone (acetone) to form an enolate ion. The enolate ion is stabilized by resonance, with the negative charge delocalized over the oxygen and carbon atoms.
2. The enolate ion reacts with iodine (I2) to form a diiodoalkoxide intermediate. This intermediate is unstable and undergoes a rearrangement to form the iodoform product.
3. The diiodoalkoxide intermediate loses an iodide ion and forms iodoform (CHI3) as the final product. The iodoform precipitates out of solution as a yellow solid.
Now, let's analyze the given options:
a) CH3COCH2I: This compound is formed in the iodoform reaction. Acetone reacts with iodine and a base to form iodoform.
b) ICH2COCH2I: This compound is NOT formed in the iodoform reaction. The presence of two iodine atoms on the second carbon atom prevents the formation of iodoform. This compound does not possess a methyl ketone functional group.
c) CH3COCHI2: This compound is formed in the iodoform reaction. Acetone reacts with iodine and a base to form iodoform.
d) CH3COCI3: This compound is NOT formed in the iodoform reaction. The presence of three chlorine atoms prevents the formation of iodoform. This compound does not possess a methyl ketone functional group.
Therefore, the correct answer is option b) ICH2COCH2I. This compound is not formed in the iodoform reaction because it does not possess a methyl ketone functional group and has two iodine atoms on the second carbon atom, which prevents the formation of iodoform.
The iodoform reaction is a test used to determine the presence of a methyl ketone or a compound with a methyl ketone functional group. In this reaction, the methyl ketone reacts with iodine and a base to form a yellow precipitate of iodoform.
The reaction mechanism involves the following steps:
1. The base (usually sodium hydroxide, NaOH) deprotonates the methyl ketone (acetone) to form an enolate ion. The enolate ion is stabilized by resonance, with the negative charge delocalized over the oxygen and carbon atoms.
2. The enolate ion reacts with iodine (I2) to form a diiodoalkoxide intermediate. This intermediate is unstable and undergoes a rearrangement to form the iodoform product.
3. The diiodoalkoxide intermediate loses an iodide ion and forms iodoform (CHI3) as the final product. The iodoform precipitates out of solution as a yellow solid.
Now, let's analyze the given options:
a) CH3COCH2I: This compound is formed in the iodoform reaction. Acetone reacts with iodine and a base to form iodoform.
b) ICH2COCH2I: This compound is NOT formed in the iodoform reaction. The presence of two iodine atoms on the second carbon atom prevents the formation of iodoform. This compound does not possess a methyl ketone functional group.
c) CH3COCHI2: This compound is formed in the iodoform reaction. Acetone reacts with iodine and a base to form iodoform.
d) CH3COCI3: This compound is NOT formed in the iodoform reaction. The presence of three chlorine atoms prevents the formation of iodoform. This compound does not possess a methyl ketone functional group.
Therefore, the correct answer is option b) ICH2COCH2I. This compound is not formed in the iodoform reaction because it does not possess a methyl ketone functional group and has two iodine atoms on the second carbon atom, which prevents the formation of iodoform.
Let a , b, c be three real numbers such that a + 2b + 4c = 0. Then the equation ax2 + bx + c = 0- a)has both the roots complex
- b)hat its roots lying within – 1 < x < 0
- c)has one of roots equal to 1/2
- d)has its roots lying within 2 < x < 6
Correct answer is option 'C'. Can you explain this answer?
Let a , b, c be three real numbers such that a + 2b + 4c = 0. Then the equation ax2 + bx + c = 0
a)
has both the roots complex
b)
hat its roots lying within – 1 < x < 0
c)
has one of roots equal to 1/2
d)
has its roots lying within 2 < x < 6
|
|
Khaja Moinuddin answered |

If the straight line y = mx lies outside of the circle x2 + y2 – 20y + 90 = 0, then the value of m will satisfy- a)m < 3
- b)|m| < 3
- c)m > 3
- d)|m| > 3
Correct answer is option 'B'. Can you explain this answer?
If the straight line y = mx lies outside of the circle x2 + y2 – 20y + 90 = 0, then the value of m will satisfy
a)
m < 3
b)
|m| < 3
c)
m > 3
d)
|m| > 3
|
|
Bhaskar Choudhury answered |
x2 +m2 x2− 20mx + 90
x2 (1 + m2)− 20mx + 90 = 0
D <0
400m2 − 4× 90 (1 + m2) < 0
40m2 < 360
m2 < 9 ; |m |< 3
A body floats in water with 40% of its volume outside water. When the same body floats in an oil, 60% of its volume remains outside oil. The relative density of oil is- a)0.9
- b)1.0
- c)1.2
- d)1.5
Correct answer is option 'D'. Can you explain this answer?
A body floats in water with 40% of its volume outside water. When the same body floats in an oil, 60% of its volume remains outside oil. The relative density of oil is
a)
0.9
b)
1.0
c)
1.2
d)
1.5
|
|
Naina Choudhury answered |
Vσg = 0.6 Vσ1g ...... (1)
Vσg = 0.4 Vσ2g .................. (2)
Dividing (1) and (2)
= 3/2
In Young’s double slit experiment the two slits are d distance apart. Interference pattern is observed on a screen at a distance D from the slits. A dark fringe is observed on the screen directly opposite to one of the slits. The wavelength of light is- a)D2/2d
- b)d2/2D
- c)D2/d
- d)d2/D
Correct answer is option 'D'. Can you explain this answer?
In Young’s double slit experiment the two slits are d distance apart. Interference pattern is observed on a screen at a distance D from the slits. A dark fringe is observed on the screen directly opposite to one of the slits. The wavelength of light is
a)
D2/2d
b)
d2/2D
c)
D2/d
d)
d2/D
|
|
Meera Nambiar answered |
The function f(x) = ax + b is strictly increasing for all real x if- a)a > 0
- b)a < 0
- c)a = 0
- d)a ≤ 0
Correct answer is option 'A'. Can you explain this answer?
The function f(x) = ax + b is strictly increasing for all real x if
a)
a > 0
b)
a < 0
c)
a = 0
d)
a ≤ 0
|
|
Bhukya Nithin answered |
As the derivative of the given function is = a,
we have to equate it to zero,it does conclude that the given expression has only one zero that is at (a)..
so the value of (a) should always be greater than zero to satisfy the condition..
we have to equate it to zero,it does conclude that the given expression has only one zero that is at (a)..
so the value of (a) should always be greater than zero to satisfy the condition..
Reaction of formaldehyde and ammonia gives- a)Hexamethylene tetramine
- b)Bakelite
- c)Urea
- d)Triethylene Tetramine
Correct answer is option 'A'. Can you explain this answer?
Reaction of formaldehyde and ammonia gives
a)
Hexamethylene tetramine
b)
Bakelite
c)
Urea
d)
Triethylene Tetramine
|
|
Madhu Pandey answered |
Formaldehyde react with ammonia to form hexamethylenetetramine. which is used as urinary antiseptic under the nate urotopine.
6HCHO + 4NH3 = (CH2)6N4 + 6H2O
6HCHO + 4NH3 = (CH2)6N4 + 6H2O
Which of the following compounds has maximum volatility?A. 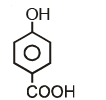 B.
B. 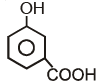 C.
C. 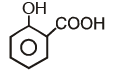 D.
D. 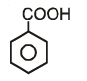
- a)A
- b)B
- c)C
- d)D
Correct answer is option 'C'. Can you explain this answer?
Which of the following compounds has maximum volatility?
A. 
B. 
C. 
D. 
a)
A
b)
B
c)
C
d)
D
|
|
Baby Ghosh answered |
Option C is correct answer because the distance of Carboxylic group and hydroxy grp is minimum. So...It is possible in only Ortho product.Not in meta and para products. And In compound IV there isn't present any another group except Carboxylic grp..So..ortho product is the correct answer.
A cubical vessel of height 1 m is full of water. What is the amount of work done in pumping water out of the vessel? (Take g = 10 m s–2)- a)1250 J
- b)5000 J
- c)1000 J
- d)2500 J
Correct answer is option 'B'. Can you explain this answer?
A cubical vessel of height 1 m is full of water. What is the amount of work done in pumping water out of the vessel? (Take g = 10 m s–2)
a)
1250 J
b)
5000 J
c)
1000 J
d)
2500 J
|
|
Manasa Das answered |
V = l3=1m3
m = 1 x 1000 = 1000kg
w = mgh = 1000 x 10 x 1/2= 5000J
1.56 × 105 J of heat is conducted through a 2 m2 wall of 12 cm thick in one hour. Temperature difference between the two sides of the wall is 200C. The thermal conductivity of the material of the wall is (in W m–1 K–1)- a)0.11
- b)0.13
- c)0.15
- d)1.2
Correct answer is option 'B'. Can you explain this answer?
1.56 × 105 J of heat is conducted through a 2 m2 wall of 12 cm thick in one hour. Temperature difference between the two sides of the wall is 200C. The thermal conductivity of the material of the wall is (in W m–1 K–1)
a)
0.11
b)
0.13
c)
0.15
d)
1.2
|
|
Tanishq Choudhary answered |
The displacement of a particle in S.H.M. varies according to the relation x = 4(cos πt + sin πt). The amplitude of the particle is- a)– 4
- b)4
- c)4√2
- d)8
Correct answer is option 'C'. Can you explain this answer?
The displacement of a particle in S.H.M. varies according to the relation x = 4(cos πt + sin πt). The amplitude of the particle is
a)
– 4
b)
4
c)
4√2
d)
8

|
Raja Kotni answered |
Option c is the answer and x=asinwt u change that eqn into above form
The intercept on the line y = x by the circle x2 + y2 – 2x = 0 is AB. Equation of the circle with AB as diameter is- a)x2 + y2= 1
- b)x(x – 1) +y(y – 1) = 0
- c)x2 + y2 = 2
- d)(x –1)(x–2)+(y–1)+(y–2)= 0
Correct answer is option 'B'. Can you explain this answer?
The intercept on the line y = x by the circle x2 + y2 – 2x = 0 is AB. Equation of the circle with AB as diameter is
a)
x2 + y2= 1
b)
x(x – 1) +y(y – 1) = 0
c)
x2 + y2 = 2
d)
(x –1)(x–2)+(y–1)+(y–2)= 0
|
|
Aaditya Datta answered |
x2 + y2 = 0
( 0, 0 ) , (1,1) as diametric ends (x − 0)(x−1) + (y + 0)(y −1) = 0
x2 +y2− x − y = 0
Chapter doubts & questions for 2011 - WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of 2011 - WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
WBJEE Sample Papers, Section Wise & Full Mock Tests 2026
3 videos|21 docs|54 tests
|
Related JEE Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup