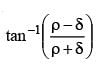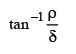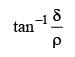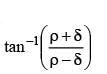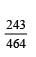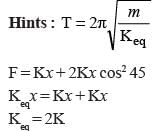All Exams >
JEE >
WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 >
All Questions
All questions of 2010 for JEE Exam
A stone falls freely from rest and the total distance covered by it in the last second of its motion equals the distance covered by it in the first three seconds of its motion. The stone remains in the air for- a)6 s
- b)5 s
- c)7 s
- d)4 s
Correct answer is option 'B'. Can you explain this answer?
A stone falls freely from rest and the total distance covered by it in the last second of its motion equals the distance covered by it in the first three seconds of its motion. The stone remains in the air for
a)
6 s
b)
5 s
c)
7 s
d)
4 s
|
|
Gaurav Kumar answered |
u = 0
What is obtained when nitrobenzene is treated sequentially with (i) NH4Cl/Zn dust and (ii) H2SO4/Na2Cr2O7 ?- a)meta-chloronitrobenzene
- b)para-chloronitrobenzene
- c)nitrosobenzene
- d)benzene
Correct answer is option 'C'. Can you explain this answer?
What is obtained when nitrobenzene is treated sequentially with (i) NH4Cl/Zn dust and (ii) H2SO4/Na2Cr2O7 ?
a)
meta-chloronitrobenzene
b)
para-chloronitrobenzene
c)
nitrosobenzene
d)
benzene
|
|
Anagha Dey answered |
Reaction Overview
When nitrobenzene is treated sequentially with (i) NH4Cl/Zn dust followed by (ii) H2SO4/Na2Cr2O7, a specific transformation occurs that leads to the formation of nitrosobenzene.
Step 1: Reduction of Nitrobenzene
- The first reagent, NH4Cl/Zn dust, serves as a reducing agent.
- Nitrobenzene (C6H5NO2) undergoes reduction to form an intermediate, aniline (C6H5NH2).
- This is a critical step as it converts the nitro group (-NO2) to an amino group (-NH2).
Step 2: Oxidation to Form Nitrosobenzene
- The next reagent, H2SO4/Na2Cr2O7, is an oxidizing agent.
- In the presence of sulfuric acid and chromium trioxide, aniline is oxidized to nitrosobenzene (C6H5NO).
- This transformation involves the formation of a diazonium salt intermediate which, upon hydrolysis and subsequent treatment, yields nitrosobenzene.
Conclusion
- The final product, nitrosobenzene, is achieved through the sequential reactions of nitrobenzene first with NH4Cl/Zn dust followed by H2SO4/Na2Cr2O7.
- Therefore, the correct answer to the question is option 'C': nitrosobenzene.
Key Points
- Nitrobenzene to aniline via reduction.
- Aniline oxidized to nitrosobenzene.
- Sequential treatment crucial for correct product formation.
When nitrobenzene is treated sequentially with (i) NH4Cl/Zn dust followed by (ii) H2SO4/Na2Cr2O7, a specific transformation occurs that leads to the formation of nitrosobenzene.
Step 1: Reduction of Nitrobenzene
- The first reagent, NH4Cl/Zn dust, serves as a reducing agent.
- Nitrobenzene (C6H5NO2) undergoes reduction to form an intermediate, aniline (C6H5NH2).
- This is a critical step as it converts the nitro group (-NO2) to an amino group (-NH2).
Step 2: Oxidation to Form Nitrosobenzene
- The next reagent, H2SO4/Na2Cr2O7, is an oxidizing agent.
- In the presence of sulfuric acid and chromium trioxide, aniline is oxidized to nitrosobenzene (C6H5NO).
- This transformation involves the formation of a diazonium salt intermediate which, upon hydrolysis and subsequent treatment, yields nitrosobenzene.
Conclusion
- The final product, nitrosobenzene, is achieved through the sequential reactions of nitrobenzene first with NH4Cl/Zn dust followed by H2SO4/Na2Cr2O7.
- Therefore, the correct answer to the question is option 'C': nitrosobenzene.
Key Points
- Nitrobenzene to aniline via reduction.
- Aniline oxidized to nitrosobenzene.
- Sequential treatment crucial for correct product formation.
Dipole moment of 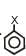 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of 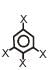 is
is- a)1.5 D
- b)2.25 D
- c)1D
- d)3D
Correct answer is option 'A'. Can you explain this answer?
Dipole moment of  is 1.5D. The dipole moment of
is 1.5D. The dipole moment of  is
is
 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of  is
isa)
1.5 D
b)
2.25 D
c)
1D
d)
3D
|
|
Mohit Mittal answered |
A is correct , as after do components of dipole due to x present on both side after solving , u will get ans.
If a species has 16 protons, 18 electrons and 16 neutrons, find the species and its charge- a)S1–
- b)Si2–
- c)P3–
- d)S2–
Correct answer is option 'D'. Can you explain this answer?
If a species has 16 protons, 18 electrons and 16 neutrons, find the species and its charge
a)
S1–
b)
Si2–
c)
P3–
d)
S2–
|
|
Mohit Mittal answered |
D is correct as atomic no. = no. of proton
The ratio of magnetic field and magnetic moment at the centre of a current carrying circular loop is x. When both the current and radius is doubled the ratio will be- a)x/8
- b)x/4
- c)x/2
- d)2x
Correct answer is option 'A'. Can you explain this answer?
The ratio of magnetic field and magnetic moment at the centre of a current carrying circular loop is x. When both the current and radius is doubled the ratio will be
a)
x/8
b)
x/4
c)
x/2
d)
2x
|
|
Nilanjan Khanna answered |
Explanation:
Answer: Option A (x/8)
- Let's consider a circular loop of radius 'r' and current 'I' flowing through it.
- At the centre of the loop, magnetic field is given by the formula B = μ₀I/2r where μ₀ is the permeability of free space.
- Magnetic moment of the loop is given by the formula M = πr²I, which is the product of current, area and number of turns.
- The ratio of magnetic field and magnetic moment at the centre of the loop is x = B/M = (μ₀I/2r)/(πr²I) = μ₀/2πr.
- Now, if we double the current and radius, new magnetic field at the centre of the loop will be B' = μ₀(2I)/(2(2r)) = μ₀I/2r.
- Similarly, new magnetic moment of the loop will be M' = π(2r)²(2I) = 4πr²I.
- Hence, the new ratio of magnetic field and magnetic moment at the centre of the loop will be x' = B'/M' = (μ₀I/2r)/(4πr²I) = μ₀/8πr.
- Therefore, the ratio of magnetic field and magnetic moment at the centre of the loop is reduced to x/8 when both the current and radius are doubled.
Answer: Option A (x/8)
For a reversible chemical reaction where the forward process is exothermic, which of the following statements is correct ?- a)The backward reaction has higher activation energy than the forward reaction
- b)The backward and the forward processes have the same activation energy
- c)The backward reaction has lower activation energy
- d)No activation anergy is required at all since energy is liberated in the process.
Correct answer is option 'A'. Can you explain this answer?
For a reversible chemical reaction where the forward process is exothermic, which of the following statements is correct ?
a)
The backward reaction has higher activation energy than the forward reaction
b)
The backward and the forward processes have the same activation energy
c)
The backward reaction has lower activation energy
d)
No activation anergy is required at all since energy is liberated in the process.
|
|
Neha Joshi answered |
The decimal equivalent of the binary number (11010.101)2 is- a)9.625
- b)25.265
- c)26.625
- d)26.265
Correct answer is option 'C'. Can you explain this answer?
The decimal equivalent of the binary number (11010.101)2 is
a)
9.625
b)
25.265
c)
26.625
d)
26.265
|
|
Puja Banerjee answered |
Decimal Equivalent of Binary Number (11010.101)2
To find the decimal equivalent of a binary number, we need to understand the place value system in binary and decimal systems.
Binary Place Values:
In a binary number, each digit represents a power of 2 starting from right to left. The rightmost digit represents 2^0, the next digit represents 2^1, the next represents 2^2, and so on.
Decimal Place Values:
In a decimal number, each digit represents a power of 10 starting from right to left. The rightmost digit represents 10^0, the next digit represents 10^1, the next represents 10^2, and so on.
Converting the Integer Part:
The integer part of the binary number (11010)2 can be converted to decimal by multiplying each digit with the corresponding power of 2 and adding them together.
(11010)2 = (1 * 2^4) + (1 * 2^3) + (0 * 2^2) + (1 * 2^1) + (0 * 2^0)
= 16 + 8 + 0 + 2 + 0
= 26
Converting the Fractional Part:
The fractional part of the binary number (0.101)2 can be converted to decimal by multiplying each digit with the corresponding negative power of 2 and adding them together.
(0.101)2 = (1 * 2^-1) + (0 * 2^-2) + (1 * 2^-3)
= 0.5 + 0 + 0.125
= 0.625
Combining the Integer and Fractional Part:
To find the decimal equivalent of the entire binary number (11010.101)2, we combine the decimal values of the integer and fractional parts.
Decimal equivalent = Integer part + Fractional part
= 26 + 0.625
= 26.625
Therefore, the correct answer is option C) 26.625.
To find the decimal equivalent of a binary number, we need to understand the place value system in binary and decimal systems.
Binary Place Values:
In a binary number, each digit represents a power of 2 starting from right to left. The rightmost digit represents 2^0, the next digit represents 2^1, the next represents 2^2, and so on.
Decimal Place Values:
In a decimal number, each digit represents a power of 10 starting from right to left. The rightmost digit represents 10^0, the next digit represents 10^1, the next represents 10^2, and so on.
Converting the Integer Part:
The integer part of the binary number (11010)2 can be converted to decimal by multiplying each digit with the corresponding power of 2 and adding them together.
(11010)2 = (1 * 2^4) + (1 * 2^3) + (0 * 2^2) + (1 * 2^1) + (0 * 2^0)
= 16 + 8 + 0 + 2 + 0
= 26
Converting the Fractional Part:
The fractional part of the binary number (0.101)2 can be converted to decimal by multiplying each digit with the corresponding negative power of 2 and adding them together.
(0.101)2 = (1 * 2^-1) + (0 * 2^-2) + (1 * 2^-3)
= 0.5 + 0 + 0.125
= 0.625
Combining the Integer and Fractional Part:
To find the decimal equivalent of the entire binary number (11010.101)2, we combine the decimal values of the integer and fractional parts.
Decimal equivalent = Integer part + Fractional part
= 26 + 0.625
= 26.625
Therefore, the correct answer is option C) 26.625.
In the hydrolysis of an organic chloride in presence of large excess of water; RCI + H2O → ROH + HCl- a)Molecularity and order of reaction both are 2
- b)Molecularity is 2 but order of reaction is 1
- c)Molecularity is 1 but order of reaction is 2
- d)Molecularity is 1 and order of reaction is also 1
Correct answer is option 'B'. Can you explain this answer?
In the hydrolysis of an organic chloride in presence of large excess of water; RCI + H2O → ROH + HCl
a)
Molecularity and order of reaction both are 2
b)
Molecularity is 2 but order of reaction is 1
c)
Molecularity is 1 but order of reaction is 2
d)
Molecularity is 1 and order of reaction is also 1
|
|
Ankita Kaur answered |
Which of the following statements regarding ozone is not correct ?- a)The Ozone molecule is angular in shape
- b)The Ozone is a resonance hybrid of two structures
- c)The Oxygen– Oxygen bond length in ozone is identical with that of molecular oxygen
- d)Ozone is used as germicide and disinfectant for the purification of air.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements regarding ozone is not correct ?
a)
The Ozone molecule is angular in shape
b)
The Ozone is a resonance hybrid of two structures
c)
The Oxygen– Oxygen bond length in ozone is identical with that of molecular oxygen
d)
Ozone is used as germicide and disinfectant for the purification of air.
|
|
Aman Verma answered |
Understanding Ozone and Its Properties
Ozone (O3) is a triatomic molecule composed of three oxygen atoms. Its unique properties and structure distinguish it from molecular oxygen (O2). Among the options provided, statement 'C' is incorrect. Here’s why:
Ozone Molecule Structure
- Ozone has an angular or bent shape due to the presence of a lone pair of electrons on the central oxygen atom.
- It forms a V-shaped structure with bond angles of approximately 117 degrees.
Bonding and Resonance
- Ozone is indeed a resonance hybrid of two structures. This means that it can be represented by multiple Lewis structures, which contribute to its actual structure.
- The resonance stabilizes the molecule and accounts for the equal bond lengths observed in ozone.
Oxygen-Oxygen Bond Length
- Statement 'C' claims that the Oxygen-Oxygen bond length in ozone is identical to that of molecular oxygen. This is incorrect.
- The bond length in ozone is longer than that in molecular oxygen due to the nature of the bonding and resonance. In O2, the bond is a double bond, while in ozone, the bond characteristics are influenced by resonance, resulting in longer bond lengths.
Applications of Ozone
- Ozone is widely used as a germicide and disinfectant. It purifies air and water, making it effective in sanitation processes.
- Its strong oxidizing properties allow it to kill bacteria and deactivate viruses, highlighting its importance in health and safety.
In conclusion, option 'C' is incorrect because the bond lengths in ozone and molecular oxygen are not the same, reflecting the unique characteristics of ozone's molecular structure.
Ozone (O3) is a triatomic molecule composed of three oxygen atoms. Its unique properties and structure distinguish it from molecular oxygen (O2). Among the options provided, statement 'C' is incorrect. Here’s why:
Ozone Molecule Structure
- Ozone has an angular or bent shape due to the presence of a lone pair of electrons on the central oxygen atom.
- It forms a V-shaped structure with bond angles of approximately 117 degrees.
Bonding and Resonance
- Ozone is indeed a resonance hybrid of two structures. This means that it can be represented by multiple Lewis structures, which contribute to its actual structure.
- The resonance stabilizes the molecule and accounts for the equal bond lengths observed in ozone.
Oxygen-Oxygen Bond Length
- Statement 'C' claims that the Oxygen-Oxygen bond length in ozone is identical to that of molecular oxygen. This is incorrect.
- The bond length in ozone is longer than that in molecular oxygen due to the nature of the bonding and resonance. In O2, the bond is a double bond, while in ozone, the bond characteristics are influenced by resonance, resulting in longer bond lengths.
Applications of Ozone
- Ozone is widely used as a germicide and disinfectant. It purifies air and water, making it effective in sanitation processes.
- Its strong oxidizing properties allow it to kill bacteria and deactivate viruses, highlighting its importance in health and safety.
In conclusion, option 'C' is incorrect because the bond lengths in ozone and molecular oxygen are not the same, reflecting the unique characteristics of ozone's molecular structure.
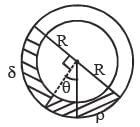
Two mirrors at an angle θº produce 5 images of a point. The number of images produced when θ is decreased to θº – 30º is- a)9
- b)10
- c)11
- d)12
Correct answer is option 'C'. Can you explain this answer?
Two mirrors at an angle θº produce 5 images of a point. The number of images produced when θ is decreased to θº – 30º is
a)
9
b)
10
c)
11
d)
12
|
|
Anaya Patel answered |
No. of images = 5
∴ θ = 60º
New angle = θ – 30º = 30º. No of images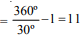
∴ θ = 60º
New angle = θ – 30º = 30º. No of images
Which of the following orders regarding ionization energy is correct ? - a)N > O > F
- b)N < O < F
- c)N > O < F
- d)N < O > F
Correct answer is option 'C'. Can you explain this answer?
Which of the following orders regarding ionization energy is correct ?
a)
N > O > F
b)
N < O < F
c)
N > O < F
d)
N < O > F
|
|
Maitri Gupta answered |
Explanation:
Ionization energy:
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. It is measured in kilojoules per mole (kJ/mol).
Trend in ionization energy:
- Ionization energy generally increases across a period from left to right and decreases down a group from top to bottom.
- This is because as you move across a period, the effective nuclear charge increases, making it harder to remove an electron. As you move down a group, the outer electrons are further away from the nucleus, making them easier to remove.
Order of ionization energy:
- In this case, the correct order is N > O < />
- Nitrogen (N) has a higher ionization energy than oxygen (O) because nitrogen has one less electron shell, making it harder to remove an electron.
- Fluorine (F) has the highest ionization energy among the three because it is the most electronegative element and has the smallest atomic radius, making it the most difficult to remove an electron from.
Therefore, the correct order of ionization energy is N > O < f.="" />
Ionization energy:
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. It is measured in kilojoules per mole (kJ/mol).
Trend in ionization energy:
- Ionization energy generally increases across a period from left to right and decreases down a group from top to bottom.
- This is because as you move across a period, the effective nuclear charge increases, making it harder to remove an electron. As you move down a group, the outer electrons are further away from the nucleus, making them easier to remove.
Order of ionization energy:
- In this case, the correct order is N > O < />
- Nitrogen (N) has a higher ionization energy than oxygen (O) because nitrogen has one less electron shell, making it harder to remove an electron.
- Fluorine (F) has the highest ionization energy among the three because it is the most electronegative element and has the smallest atomic radius, making it the most difficult to remove an electron from.
Therefore, the correct order of ionization energy is N > O < f.="" />
A boy of mass 40 kg is climbing a vertical pole at a constant speed. If the coefficient of friction between his palms and the poleis 0.8 and g = 10 m/s2, the horizontal force that he is applying on the pole is- a)300 N
- b)400 N
- c)500 N
- d)600 N
Correct answer is option 'C'. Can you explain this answer?
A boy of mass 40 kg is climbing a vertical pole at a constant speed. If the coefficient of friction between his palms and the poleis 0.8 and g = 10 m/s2, the horizontal force that he is applying on the pole is
a)
300 N
b)
400 N
c)
500 N
d)
600 N
|
|
Dev Kumar answered |
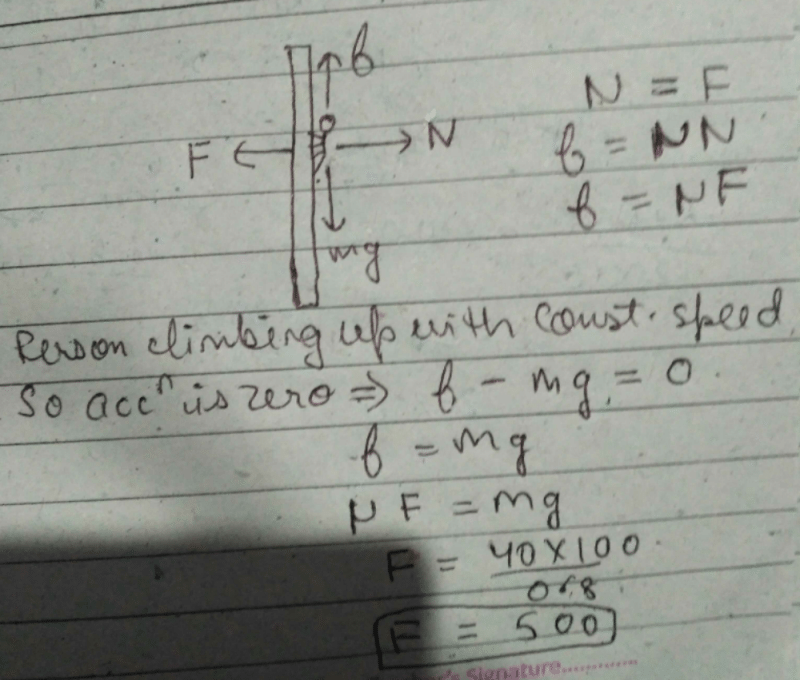
The energy released by the fission of one uranium atom is 200 MeV. The number of fissions per second required to produce 3.2 W of power is (Take 1 eV = 1.6 × 10–19 J)- a)107
- b)1010
- c)1015
- d)1011
Correct answer is option 'D'. Can you explain this answer?
The energy released by the fission of one uranium atom is 200 MeV. The number of fissions per second required to produce 3.2 W of power is (Take 1 eV = 1.6 × 10–19 J)
a)
107
b)
1010
c)
1015
d)
1011
|
|
Harshad Unni answered |
u = 200 MeV = 200 × 106 eV = 200 × 106 × 1.6 × 10–19 J
E = 3.2 J
No of fissions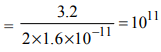
E = 3.2 J
No of fissions
A point charge +q is placed at the centre of a cube of side L. The electric flux emerging from the cube is- a)
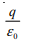
- b)Zero
- c)
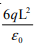
- d)
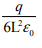
Correct answer is option 'A'. Can you explain this answer?
A point charge +q is placed at the centre of a cube of side L. The electric flux emerging from the cube is
a)
b)
Zero
c)
d)
|
|
Chirag Verma answered |
Concept: Gauss Law.By Gauss theorem ...Total electric flux = Total charge inside cube /€. Flux = q/€. (where € is permittivity). Hence answer is 'A'.
Ortho-and para-hydrogens have- a)Identical chemical properties but different physical properties
- b)Identical physical and chemical properties
- c)Identical physical properties but different chemical properties
- d)Different physical and chemical properties
Correct answer is option 'A'. Can you explain this answer?
Ortho-and para-hydrogens have
a)
Identical chemical properties but different physical properties
b)
Identical physical and chemical properties
c)
Identical physical properties but different chemical properties
d)
Different physical and chemical properties
|
|
Atharva Gupta answered |
Introduction to Ortho and Para-Hydrogens
Ortho-hydrogen and para-hydrogen are two different spin isomers of molecular hydrogen (H2). Their distinction arises from the alignment of the nuclear spins of the two hydrogen atoms.
Identical Chemical Properties
- Both ortho and para-hydrogen consist of the same molecular formula (H2), meaning they have the same chemical composition.
- Consequently, they exhibit identical chemical properties, such as reactivity and types of chemical bonds formed in reactions.
Different Physical Properties
- Spin States: In ortho-hydrogen, both hydrogen nuclei have parallel spins, while in para-hydrogen, the spins are anti-parallel. This difference in spin states influences their physical behavior.
- Energy Levels: Ortho-hydrogen is higher in energy than para-hydrogen due to its spin configuration. As a result, ortho-hydrogen can convert to para-hydrogen and vice versa, but under normal conditions, they exist in different proportions.
- Thermal Properties: The physical properties such as boiling points and heat capacities differ. Para-hydrogen has a lower boiling point and is denser than ortho-hydrogen.
Conclusion
In summary, ortho and para-hydrogen share identical chemical properties due to their same molecular structure. However, their different spin states lead to variations in physical properties, making option 'A' the correct choice. Understanding these distinctions is crucial in fields like cryogenics and quantum mechanics.
Ortho-hydrogen and para-hydrogen are two different spin isomers of molecular hydrogen (H2). Their distinction arises from the alignment of the nuclear spins of the two hydrogen atoms.
Identical Chemical Properties
- Both ortho and para-hydrogen consist of the same molecular formula (H2), meaning they have the same chemical composition.
- Consequently, they exhibit identical chemical properties, such as reactivity and types of chemical bonds formed in reactions.
Different Physical Properties
- Spin States: In ortho-hydrogen, both hydrogen nuclei have parallel spins, while in para-hydrogen, the spins are anti-parallel. This difference in spin states influences their physical behavior.
- Energy Levels: Ortho-hydrogen is higher in energy than para-hydrogen due to its spin configuration. As a result, ortho-hydrogen can convert to para-hydrogen and vice versa, but under normal conditions, they exist in different proportions.
- Thermal Properties: The physical properties such as boiling points and heat capacities differ. Para-hydrogen has a lower boiling point and is denser than ortho-hydrogen.
Conclusion
In summary, ortho and para-hydrogen share identical chemical properties due to their same molecular structure. However, their different spin states lead to variations in physical properties, making option 'A' the correct choice. Understanding these distinctions is crucial in fields like cryogenics and quantum mechanics.
Chapter doubts & questions for 2010 - WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of 2010 - WBJEE Sample Papers, Section Wise & Full Mock Tests 2026 in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
WBJEE Sample Papers, Section Wise & Full Mock Tests 2026
3 videos|21 docs|54 tests
|
Related JEE Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily