All Exams >
JEE >
VITEEE: Subject Wise and Full Length MOCK Tests >
All Questions
All questions of Chemistry for JEE Exam
Same amount of electric current is passed through solutions of AgNO₃ and HCI. If 1.08 g of silver is obtained in the first case, the volume of hydrogen liberated at S.T.P. in the second case is- a)22400 ml
- b)2240 ml
- c)224 ml
- d)112 ml
Correct answer is option 'D'. Can you explain this answer?
Same amount of electric current is passed through solutions of AgNO₃ and HCI. If 1.08 g of silver is obtained in the first case, the volume of hydrogen liberated at S.T.P. in the second case is
a)
22400 ml
b)
2240 ml
c)
224 ml
d)
112 ml
|
|
Neha Joshi answered |
Number of equivalent for Ag =( 1.08/108 )=0.01eq
Since 1 eq. produces 11.2 litre of H2 at STP
So 0.01 eq. produces (11.2�0.01) litre of H2
Or vol=0.112 litre
=112ml
A Carnot engine, working between 300 K to 600 K has a work output of 800 J-cycle⁻1. What is the amount of heat energy supplied to the engine from the source per cycle?
- a)6 KJ
- b)4 KJ
- c)1.6KJ
- d)1 KJ
Correct answer is option 'C'. Can you explain this answer?
A Carnot engine, working between 300 K to 600 K has a work output of 800 J-cycle⁻1. What is the amount of heat energy supplied to the engine from the source per cycle?
a)
6 KJ
b)
4 KJ
c)
1.6KJ
d)
1 KJ
|
|
Aditya Kulkarni answered |
Given:
Temperature of source, T1 = 500 K
Temperature of sink, T2 = 300 K
Work output, W = 800 J/cycle
To find: Heat energy supplied to the engine from the source per cycle
Formula used: Efficiency of Carnot engine = (T1-T2)/T1
Efficiency of Carnot engine can also be expressed as:
Efficiency = Work output / Heat input
Heat input = Work output / Efficiency
Calculation:
Efficiency of Carnot engine = (T1-T2)/T1 = (500-300)/500 = 0.4 or 40%
Heat input = Work output / Efficiency = 800 / 0.4 = 2000 J/cycle
Therefore, the amount of heat energy supplied to the engine from the source per cycle is 2 KJ (2000 J/cycle).
Answer: c) 2 KJ
Temperature of source, T1 = 500 K
Temperature of sink, T2 = 300 K
Work output, W = 800 J/cycle
To find: Heat energy supplied to the engine from the source per cycle
Formula used: Efficiency of Carnot engine = (T1-T2)/T1
Efficiency of Carnot engine can also be expressed as:
Efficiency = Work output / Heat input
Heat input = Work output / Efficiency
Calculation:
Efficiency of Carnot engine = (T1-T2)/T1 = (500-300)/500 = 0.4 or 40%
Heat input = Work output / Efficiency = 800 / 0.4 = 2000 J/cycle
Therefore, the amount of heat energy supplied to the engine from the source per cycle is 2 KJ (2000 J/cycle).
Answer: c) 2 KJ
Which one of the following is an example of exothermic reaction?- a)H2 (g) + Cl2 (g) → 2HCl(g) ΔH = − 184. 6 kJ
- b)N2(g) + O2(g) → 2NO(g) ,ΔH = + 180.8 k J
- c)C(graphite)+ H2O(g) → CO(g) + H2 (g) − 131.4 kJ
- d)C(graphite)+ 2S(s) + 91.9 kJ → CS2(l)
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is an example of exothermic reaction?
a)
H2 (g) + Cl2 (g) → 2HCl(g) ΔH = − 184. 6 kJ
b)
N2(g) + O2(g) → 2NO(g) ,ΔH = + 180.8 k J
c)
C(graphite)+ H2O(g) → CO(g) + H2 (g) − 131.4 kJ
d)
C(graphite)+ 2S(s) + 91.9 kJ → CS2(l)
|
|
Rajeev Choudhary answered |
B)2H2O(l) → 2H2(g) + O2(g)
The brown ring complex compounds is formulated as [Fe(H2O)5NO] SO4. The oxidation state of iron is
- a)+2
- b)+1
- c)+3
- d)0
Correct answer is option 'B'. Can you explain this answer?
The brown ring complex compounds is formulated as [Fe(H2O)5NO] SO4. The oxidation state of iron is
a)
+2
b)
+1
c)
+3
d)
0
|
|
Anu Dey answered |
The brown ring complex compound [Fe(H2O)5NO]SO4 has the following components:
1. Iron (Fe)
2. Water molecules (H2O)
3. Nitric oxide (NO)
4. Sulfate ion (SO4)
To determine the oxidation state of iron in this compound, we need to first understand the concept of oxidation state.
Oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. It is also known as oxidation number.
To determine the oxidation state of iron in this compound, we need to assign oxidation states to all the other atoms in the compound first.
Oxidation state of oxygen (O) in water (H2O) is -2.
Oxidation state of nitrogen (N) in nitric oxide (NO) is +2.
Oxidation state of sulfur (S) in sulfate ion (SO4) is +6.
Using this information, we can calculate the oxidation state of iron as follows:
Let x be the oxidation state of iron (Fe).
The total charge of the compound must be zero, so we can write:
(+5) x [(oxidation state of Fe)] + (-10) x [(oxidation state of O in H2O)] + (2) x [(oxidation state of N in NO)] + (-2) x [(oxidation state of S in SO4)] = 0
Simplifying the above equation, we get:
(+5)x + (-20) + (2) x (+2) + (-2) x (+6) = 0
(+5)x - 20 + 4 - 12 = 0
(+5)x - 28 = 0
(+5)x = 28
x = +5.6
Since the oxidation state of iron cannot be a fractional number, we need to round it to the nearest whole number. In this case, the oxidation state of iron is +6.
However, we need to take into account the fact that Fe is coordinated to five water molecules and one NO molecule. The sum of the oxidation states of all ligands in a complex is equal to the charge of the complex. In this case, the complex has a charge of +1 (from the sulfate ion), so the sum of the oxidation states of the ligands must be -1.
Water molecules are neutral, so their oxidation state is 0.
The oxidation state of NO is +2, so the sum of the oxidation states of the five water molecules must be -2.
Therefore, the sum of the oxidation states of the water molecules and the NO molecule is:
(5 x 0) + (+2) = +2
Since the sum of the oxidation states of the ligands is -1, the oxidation state of iron must be:
-1 - (+2) = -3
However, we need to remember that the oxidation state of iron in a complex is not always the same as its formal oxidation state. In this case, the iron atom is in a low-spin state, which means that the d electrons are paired up in the t2g orbitals. This indicates that the iron atom has lost two electrons, which corresponds to an oxidation state of +2.
Therefore, the oxidation state of iron in the brown ring complex compound [Fe(H2O)5NO]
1. Iron (Fe)
2. Water molecules (H2O)
3. Nitric oxide (NO)
4. Sulfate ion (SO4)
To determine the oxidation state of iron in this compound, we need to first understand the concept of oxidation state.
Oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. It is also known as oxidation number.
To determine the oxidation state of iron in this compound, we need to assign oxidation states to all the other atoms in the compound first.
Oxidation state of oxygen (O) in water (H2O) is -2.
Oxidation state of nitrogen (N) in nitric oxide (NO) is +2.
Oxidation state of sulfur (S) in sulfate ion (SO4) is +6.
Using this information, we can calculate the oxidation state of iron as follows:
Let x be the oxidation state of iron (Fe).
The total charge of the compound must be zero, so we can write:
(+5) x [(oxidation state of Fe)] + (-10) x [(oxidation state of O in H2O)] + (2) x [(oxidation state of N in NO)] + (-2) x [(oxidation state of S in SO4)] = 0
Simplifying the above equation, we get:
(+5)x + (-20) + (2) x (+2) + (-2) x (+6) = 0
(+5)x - 20 + 4 - 12 = 0
(+5)x - 28 = 0
(+5)x = 28
x = +5.6
Since the oxidation state of iron cannot be a fractional number, we need to round it to the nearest whole number. In this case, the oxidation state of iron is +6.
However, we need to take into account the fact that Fe is coordinated to five water molecules and one NO molecule. The sum of the oxidation states of all ligands in a complex is equal to the charge of the complex. In this case, the complex has a charge of +1 (from the sulfate ion), so the sum of the oxidation states of the ligands must be -1.
Water molecules are neutral, so their oxidation state is 0.
The oxidation state of NO is +2, so the sum of the oxidation states of the five water molecules must be -2.
Therefore, the sum of the oxidation states of the water molecules and the NO molecule is:
(5 x 0) + (+2) = +2
Since the sum of the oxidation states of the ligands is -1, the oxidation state of iron must be:
-1 - (+2) = -3
However, we need to remember that the oxidation state of iron in a complex is not always the same as its formal oxidation state. In this case, the iron atom is in a low-spin state, which means that the d electrons are paired up in the t2g orbitals. This indicates that the iron atom has lost two electrons, which corresponds to an oxidation state of +2.
Therefore, the oxidation state of iron in the brown ring complex compound [Fe(H2O)5NO]
The packing fraction for a body centred cubic is
- a)0.42
- b)0.53
- c)0.68
- d)0.82
Correct answer is option 'C'. Can you explain this answer?
The packing fraction for a body centred cubic is
a)
0.42
b)
0.53
c)
0.68
d)
0.82
|
|
Shreya Chakraborty answered |
Body Centred Cubic (BCC) Structure
The body-centred cubic (BCC) structure is one of the crystal structures commonly found in metals such as iron, chromium, and tungsten. In this structure, the central atom is surrounded by eight atoms at the corners of a cube and six atoms in the faces of the cube.
Packing Fraction
The packing fraction is the ratio of the total volume occupied by the spheres to the volume of the unit cell. The unit cell is the smallest repeating unit of a crystal lattice.
Calculation of Packing Fraction for BCC
In a BCC structure, the coordination number is 8, which means there are 8 atoms in the unit cell. The atoms at the corners of the cube contribute only 1/8th of their volume to the unit cell, and the atom in the centre contributes its full volume.
- The volume of the unit cell is calculated as V = a^3, where a is the length of the edge of the cube.
- The volume of the sphere is calculated as V = (4/3)πr^3, where r is the radius of the sphere.
The packing fraction for BCC is calculated as follows:
- Total volume of the 8 corner atoms = 8 × (1/8) × (4/3)πr^3 = (4/3)πr^3
- Volume of the central atom = (4/3)πr^3
- Total volume of the unit cell = a^3
- Packing fraction = (Volume of all atoms) / (Volume of unit cell)
- Packing fraction = 2 × [(4/3)πr^3] / a^3
Substituting for the radius of the sphere in terms of the edge length, we get:
- r = a / (2√3)
- Packing fraction = 2 × [(4/3)π(a/2√3)^3] / a^3
- Packing fraction = 2 × [(4/3)π(√3/8)a^3] / a^3
- Packing fraction = 2 × (1/6)√3π
- Packing fraction = 0.68
Therefore, the packing fraction for a body-centred cubic structure is 0.68.
The body-centred cubic (BCC) structure is one of the crystal structures commonly found in metals such as iron, chromium, and tungsten. In this structure, the central atom is surrounded by eight atoms at the corners of a cube and six atoms in the faces of the cube.
Packing Fraction
The packing fraction is the ratio of the total volume occupied by the spheres to the volume of the unit cell. The unit cell is the smallest repeating unit of a crystal lattice.
Calculation of Packing Fraction for BCC
In a BCC structure, the coordination number is 8, which means there are 8 atoms in the unit cell. The atoms at the corners of the cube contribute only 1/8th of their volume to the unit cell, and the atom in the centre contributes its full volume.
- The volume of the unit cell is calculated as V = a^3, where a is the length of the edge of the cube.
- The volume of the sphere is calculated as V = (4/3)πr^3, where r is the radius of the sphere.
The packing fraction for BCC is calculated as follows:
- Total volume of the 8 corner atoms = 8 × (1/8) × (4/3)πr^3 = (4/3)πr^3
- Volume of the central atom = (4/3)πr^3
- Total volume of the unit cell = a^3
- Packing fraction = (Volume of all atoms) / (Volume of unit cell)
- Packing fraction = 2 × [(4/3)πr^3] / a^3
Substituting for the radius of the sphere in terms of the edge length, we get:
- r = a / (2√3)
- Packing fraction = 2 × [(4/3)π(a/2√3)^3] / a^3
- Packing fraction = 2 × [(4/3)π(√3/8)a^3] / a^3
- Packing fraction = 2 × (1/6)√3π
- Packing fraction = 0.68
Therefore, the packing fraction for a body-centred cubic structure is 0.68.
When the densities of the ore particles and gangue particles are different, then the ore is concentrated by- a)hand-picking
- b)electomagnetic
- c)froth-flotation process
- d)gravity separation method
Correct answer is option 'D'. Can you explain this answer?
a)
hand-picking
b)
electomagnetic
c)
froth-flotation process
d)
gravity separation method
|
|
Hitakshi Tamta answered |
Gravity separation process, also called Hydraulic washing is based on the difference between the densities of the ore particle and gangue. It is used for the concentration of denser ores from the water soluble and lighter impurities (gangue). This method concentrates the ore by passing it through an upward stream of water whereby all the lighter particles of gangue are separated from the heavier metal ore. Hence, the correct answer is option 'D'.
Phosphorus has the oxidation state of +3 in- a)Orthophosphoric acid
- b)Phosphorous acid
- c)Metaphosphoric acid
- d)Pyrophosphoric acid
Correct answer is option 'B'. Can you explain this answer?
a)
Orthophosphoric acid
b)
Phosphorous acid
c)
Metaphosphoric acid
d)
Pyrophosphoric acid
|
|
Rohit Kumar answered |
Explanation:
Oxidation state or oxidation number is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are assigned to atoms in order to help determine the redox reactions and the electronegativity of an atom.
In this question, we are asked to find out the oxidation state of phosphorus in different acids.
a) Orthophosphoric acid: The molecular formula of orthophosphoric acid is H3PO4. Orthophosphoric acid is a triprotic acid. The oxidation state of each oxygen atom in orthophosphoric acid is -2. Since there are four oxygen atoms in the molecule, the total oxidation number contributed by oxygen is -8. The oxidation state of hydrogen is +1. Therefore, the oxidation state of phosphorus can be calculated as follows:
(3 x (+1)) + (x) + (-8) = 0
Solving for x, we get x = +5
Therefore, the oxidation state of phosphorus in orthophosphoric acid is +5.
b) Phosphorous acid: The molecular formula of phosphorous acid is H3PO3. Phosphorous acid is a diprotic acid. The oxidation state of each oxygen atom in phosphorous acid is -2. Since there are three oxygen atoms in the molecule, the total oxidation number contributed by oxygen is -6. The oxidation state of hydrogen is +1. Therefore, the oxidation state of phosphorus can be calculated as follows:
(2 x (+1)) + (x) + (-6) = 0
Solving for x, we get x = +3
Therefore, the oxidation state of phosphorus in phosphorous acid is +3.
c) Metaphosphoric acid: The molecular formula of metaphosphoric acid is HPO3. Metaphosphoric acid is a monoprotic acid. The oxidation state of each oxygen atom in metaphosphoric acid is -2. Since there are three oxygen atoms in the molecule, the total oxidation number contributed by oxygen is -6. The oxidation state of hydrogen is +1. Therefore, the oxidation state of phosphorus can be calculated as follows:
(+1) + (x) + (-6) = 0
Solving for x, we get x = +5
Therefore, the oxidation state of phosphorus in metaphosphoric acid is +5.
d) Pyrophosphoric acid: The molecular formula of pyrophosphoric acid is H4P2O7. Pyrophosphoric acid is a diprotic acid. The oxidation state of each oxygen atom in pyrophosphoric acid is -2. Since there are seven oxygen atoms in the molecule, the total oxidation number contributed by oxygen is -14. The oxidation state of hydrogen is +1. Therefore, the oxidation state of phosphorus can be calculated as follows:
(2 x (+1)) + (2x) + (-14) = 0
Solving for x, we get x = +5
Therefore, the oxidation state of phosphorus in pyrophosphoric acid is +5.
Therefore, the correct answer is option B, phosphorous acid, with an oxidation state of +3.
Oxidation state or oxidation number is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are assigned to atoms in order to help determine the redox reactions and the electronegativity of an atom.
In this question, we are asked to find out the oxidation state of phosphorus in different acids.
a) Orthophosphoric acid: The molecular formula of orthophosphoric acid is H3PO4. Orthophosphoric acid is a triprotic acid. The oxidation state of each oxygen atom in orthophosphoric acid is -2. Since there are four oxygen atoms in the molecule, the total oxidation number contributed by oxygen is -8. The oxidation state of hydrogen is +1. Therefore, the oxidation state of phosphorus can be calculated as follows:
(3 x (+1)) + (x) + (-8) = 0
Solving for x, we get x = +5
Therefore, the oxidation state of phosphorus in orthophosphoric acid is +5.
b) Phosphorous acid: The molecular formula of phosphorous acid is H3PO3. Phosphorous acid is a diprotic acid. The oxidation state of each oxygen atom in phosphorous acid is -2. Since there are three oxygen atoms in the molecule, the total oxidation number contributed by oxygen is -6. The oxidation state of hydrogen is +1. Therefore, the oxidation state of phosphorus can be calculated as follows:
(2 x (+1)) + (x) + (-6) = 0
Solving for x, we get x = +3
Therefore, the oxidation state of phosphorus in phosphorous acid is +3.
c) Metaphosphoric acid: The molecular formula of metaphosphoric acid is HPO3. Metaphosphoric acid is a monoprotic acid. The oxidation state of each oxygen atom in metaphosphoric acid is -2. Since there are three oxygen atoms in the molecule, the total oxidation number contributed by oxygen is -6. The oxidation state of hydrogen is +1. Therefore, the oxidation state of phosphorus can be calculated as follows:
(+1) + (x) + (-6) = 0
Solving for x, we get x = +5
Therefore, the oxidation state of phosphorus in metaphosphoric acid is +5.
d) Pyrophosphoric acid: The molecular formula of pyrophosphoric acid is H4P2O7. Pyrophosphoric acid is a diprotic acid. The oxidation state of each oxygen atom in pyrophosphoric acid is -2. Since there are seven oxygen atoms in the molecule, the total oxidation number contributed by oxygen is -14. The oxidation state of hydrogen is +1. Therefore, the oxidation state of phosphorus can be calculated as follows:
(2 x (+1)) + (2x) + (-14) = 0
Solving for x, we get x = +5
Therefore, the oxidation state of phosphorus in pyrophosphoric acid is +5.
Therefore, the correct answer is option B, phosphorous acid, with an oxidation state of +3.
Adsorption due to strong chemical forces is called- a)chemisorption
- b)physiosorption
- c)reversible adsorption
- d)both 1 and 2
Correct answer is option 'A'. Can you explain this answer?
Adsorption due to strong chemical forces is called
a)
chemisorption
b)
physiosorption
c)
reversible adsorption
d)
both 1 and 2
|
|
Rounak Chakraborty answered |
Adsorption due to strong chemical forces is called chemisorption.
Explanation:
Chemisorption is a type of adsorption where the adsorbate species forms a chemical bond with the surface atoms or molecules of the adsorbent. It is characterized by a strong interaction between the adsorbate and the adsorbent, which leads to the formation of a chemical compound at the interface. The chemical bond formed during chemisorption is usually covalent, ionic or metallic in nature, depending on the nature of the adsorbate and the adsorbent.
Physiosorption, on the other hand, is a type of adsorption where the adsorbate species is held on the surface of the adsorbent by weak van der Waals forces. This type of adsorption is characterized by a weak interaction between the adsorbate and the adsorbent, which leads to the formation of a physical adsorption layer at the interface.
Reversible adsorption refers to the adsorption process where the adsorbate species can be desorbed from the surface of the adsorbent by changing the conditions such as temperature, pressure or concentration. Chemisorption and physiosorption can both be reversible or irreversible, depending on the nature of the adsorbate and the adsorbent.
Therefore, option A is the correct answer as chemisorption is the type of adsorption due to strong chemical forces.
Explanation:
Chemisorption is a type of adsorption where the adsorbate species forms a chemical bond with the surface atoms or molecules of the adsorbent. It is characterized by a strong interaction between the adsorbate and the adsorbent, which leads to the formation of a chemical compound at the interface. The chemical bond formed during chemisorption is usually covalent, ionic or metallic in nature, depending on the nature of the adsorbate and the adsorbent.
Physiosorption, on the other hand, is a type of adsorption where the adsorbate species is held on the surface of the adsorbent by weak van der Waals forces. This type of adsorption is characterized by a weak interaction between the adsorbate and the adsorbent, which leads to the formation of a physical adsorption layer at the interface.
Reversible adsorption refers to the adsorption process where the adsorbate species can be desorbed from the surface of the adsorbent by changing the conditions such as temperature, pressure or concentration. Chemisorption and physiosorption can both be reversible or irreversible, depending on the nature of the adsorbate and the adsorbent.
Therefore, option A is the correct answer as chemisorption is the type of adsorption due to strong chemical forces.
The rate of diffusion of methane at a given temperature is twice that of a gas X. The molecular weight of X is- a)64.0
- b)32.0
- c)4.0
- d)8.0
Correct answer is option 'A'. Can you explain this answer?
a)
64.0
b)
32.0
c)
4.0
d)
8.0
|
|
Rahul Bansal answered |
Let rate of diffusion of gas x, r1 = a
Therefore, rate of diffusion of methane,r2 = 2 a
According to Graham's Law of Diffusion

The compound which reacts with Fehling solution is- a)C₆H₅COOH
- b)HCOOH
- c)CH₃COCH₃
- d)CH₂Cl - CH₂Cl
Correct answer is option 'B'. Can you explain this answer?
The compound which reacts with Fehling solution is
a)
C₆H₅COOH
b)
HCOOH
c)
CH₃COCH₃
d)
CH₂Cl - CH₂Cl
|
|
Samridhi Deshpande answered |
Fehling's solution is used to test for the presence of reducing sugars. When a reducing sugar is present, it will react with Fehling's solution to produce a red precipitate of Cu2O.
Explanation:
- Fehling's solution is a mixture of two solutions: Fehling's A, which is a solution of copper(II) sulfate, and Fehling's B, which is a solution of sodium potassium tartrate and sodium hydroxide.
- When a reducing sugar is added to Fehling's solution and heated, it undergoes oxidation and reduces the copper(II) ions in Fehling's A to copper(I) ions, which then react with the tartrate ions in Fehling's B to form Cu2O.
- Only compounds that have a free aldehyde or ketone group can act as reducing sugars and react with Fehling's solution.
- Formic acid (HCOOH) is a reducing sugar because it has a free aldehyde group, and thus it will react with Fehling's solution to produce a red precipitate of Cu2O.
- The other compounds listed, including acetic acid (CH3COOH), acetone (CH3COCH3), and dichloromethane (CH2Cl2), do not have a free aldehyde or ketone group and therefore cannot act as reducing sugars and will not react with Fehling's solution.
Explanation:
- Fehling's solution is a mixture of two solutions: Fehling's A, which is a solution of copper(II) sulfate, and Fehling's B, which is a solution of sodium potassium tartrate and sodium hydroxide.
- When a reducing sugar is added to Fehling's solution and heated, it undergoes oxidation and reduces the copper(II) ions in Fehling's A to copper(I) ions, which then react with the tartrate ions in Fehling's B to form Cu2O.
- Only compounds that have a free aldehyde or ketone group can act as reducing sugars and react with Fehling's solution.
- Formic acid (HCOOH) is a reducing sugar because it has a free aldehyde group, and thus it will react with Fehling's solution to produce a red precipitate of Cu2O.
- The other compounds listed, including acetic acid (CH3COOH), acetone (CH3COCH3), and dichloromethane (CH2Cl2), do not have a free aldehyde or ketone group and therefore cannot act as reducing sugars and will not react with Fehling's solution.
Which of the following reagent react differently with HCHO and CH₃CHO and CH₃COCH₃- a)HCN
- b)NH₂-NH₂
- c)NH₂OH
- d)NH₃
Correct answer is option 'D'. Can you explain this answer?
Which of the following reagent react differently with HCHO and CH₃CHO and CH₃COCH₃
a)
HCN
b)
NH₂-NH₂
c)
NH₂OH
d)
NH₃
|
|
Poulomi Gupta answered |
Reactivity of Reagents with HCHO, CH3CHO, and CH3COCH3
To determine which reagent reacts differently with HCHO, CH3CHO, and CH3COCH3, we need to consider the functional groups present in these compounds and the reactions that can occur with different reagents.
HCHO: Formaldehyde (HCHO) contains an aldehyde functional group (-CHO).
CH3CHO: Acetaldehyde (CH3CHO) also contains an aldehyde functional group (-CHO).
CH3COCH3: Acetone (CH3COCH3) contains a ketone functional group (-C=O-).
Now, let's examine the reactivity of the given reagents (HCN, NH2NH2, NHOH, NH) with these compounds.
a) HCN: Hydrogen cyanide (HCN) is a weak acid and can react with aldehydes and ketones to form cyanohydrins. Both HCHO and CH3CHO can react with HCN to form cyanohydrins.
b) NH2NH2: Hydrazine (NH2NH2) is a strong reducing agent and can react with aldehydes and ketones to form hydrazones. Both HCHO and CH3CHO can react with NH2NH2 to form hydrazones.
c) NHOH: Hydroxylamine (NHOH) is a weak base and can react with aldehydes and ketones to form oximes. Both HCHO and CH3CHO can react with NHOH to form oximes.
d) NH: Ammonia (NH) is a weak base and does not react directly with aldehydes or ketones. However, it can undergo a reaction called the Hofmann degradation with primary amides. Since none of the given compounds are primary amides, NH will not react with HCHO, CH3CHO, or CH3COCH3.
Therefore, the correct answer is option 'D' - NH. NH does not react with HCHO, CH3CHO, or CH3COCH3, while the other reagents (HCN, NH2NH2, NHOH) can react with all three compounds.
To determine which reagent reacts differently with HCHO, CH3CHO, and CH3COCH3, we need to consider the functional groups present in these compounds and the reactions that can occur with different reagents.
HCHO: Formaldehyde (HCHO) contains an aldehyde functional group (-CHO).
CH3CHO: Acetaldehyde (CH3CHO) also contains an aldehyde functional group (-CHO).
CH3COCH3: Acetone (CH3COCH3) contains a ketone functional group (-C=O-).
Now, let's examine the reactivity of the given reagents (HCN, NH2NH2, NHOH, NH) with these compounds.
a) HCN: Hydrogen cyanide (HCN) is a weak acid and can react with aldehydes and ketones to form cyanohydrins. Both HCHO and CH3CHO can react with HCN to form cyanohydrins.
b) NH2NH2: Hydrazine (NH2NH2) is a strong reducing agent and can react with aldehydes and ketones to form hydrazones. Both HCHO and CH3CHO can react with NH2NH2 to form hydrazones.
c) NHOH: Hydroxylamine (NHOH) is a weak base and can react with aldehydes and ketones to form oximes. Both HCHO and CH3CHO can react with NHOH to form oximes.
d) NH: Ammonia (NH) is a weak base and does not react directly with aldehydes or ketones. However, it can undergo a reaction called the Hofmann degradation with primary amides. Since none of the given compounds are primary amides, NH will not react with HCHO, CH3CHO, or CH3COCH3.
Therefore, the correct answer is option 'D' - NH. NH does not react with HCHO, CH3CHO, or CH3COCH3, while the other reagents (HCN, NH2NH2, NHOH) can react with all three compounds.
An electrolytic cell contains a solution of Ag₂SO₄ and has Pt electrodes.A current is passed till 1.6 g of O₂ has been liberated at anode. The amount of silver deposited at cathode will be- a)107.88 g
- b)1.6 g
- c)0.8 g
- d)21.60 g
Correct answer is option 'D'. Can you explain this answer?
An electrolytic cell contains a solution of Ag₂SO₄ and has Pt electrodes.A current is passed till 1.6 g of O₂ has been liberated at anode. The amount of silver deposited at cathode will be
a)
107.88 g
b)
1.6 g
c)
0.8 g
d)
21.60 g
|
|
Tushar Jain answered |
Given:
Solution contains AgSO4
Pt electrodes are used
1.6 g of O2 is liberated at anode
To find:
Amount of silver deposited at cathode
Solution:
The given electrolysis reaction is:
2Ag+ + SO42- → Ag2SO4 + 2e- (reduction at cathode)
2H2O → O2 + 4H+ + 4e- (oxidation at anode)
From the given data, we can calculate the number of electrons passed during the electrolysis.
Oxygen gas is liberated at anode, so the number of electrons required for its formation can be calculated as follows:
1 mole of O2 requires 4 electrons
32 g of O2 requires 4 * 6.022 * 10^23 electrons
1.6 g of O2 requires 4 * 6.022 * 10^23 * 1.6 / 32 = 1.205 * 10^22 electrons
Since each Ag+ ion requires 1 electron for reduction to Ag, the number of Ag+ ions reduced can be calculated as follows:
1 mole of Ag+ requires 1 mole of electrons
107.87 g of Ag+ requires 1 * 6.022 * 10^23 electrons
1.205 * 10^22 electrons will reduce 1.205 * 10^22 / 6.022 * 10^23 * 107.87 = 0.216 g of Ag+
Therefore, the amount of silver deposited at cathode is 0.216 g or approximately 0.22 g.
The correct option is D.
Solution contains AgSO4
Pt electrodes are used
1.6 g of O2 is liberated at anode
To find:
Amount of silver deposited at cathode
Solution:
The given electrolysis reaction is:
2Ag+ + SO42- → Ag2SO4 + 2e- (reduction at cathode)
2H2O → O2 + 4H+ + 4e- (oxidation at anode)
From the given data, we can calculate the number of electrons passed during the electrolysis.
Oxygen gas is liberated at anode, so the number of electrons required for its formation can be calculated as follows:
1 mole of O2 requires 4 electrons
32 g of O2 requires 4 * 6.022 * 10^23 electrons
1.6 g of O2 requires 4 * 6.022 * 10^23 * 1.6 / 32 = 1.205 * 10^22 electrons
Since each Ag+ ion requires 1 electron for reduction to Ag, the number of Ag+ ions reduced can be calculated as follows:
1 mole of Ag+ requires 1 mole of electrons
107.87 g of Ag+ requires 1 * 6.022 * 10^23 electrons
1.205 * 10^22 electrons will reduce 1.205 * 10^22 / 6.022 * 10^23 * 107.87 = 0.216 g of Ag+
Therefore, the amount of silver deposited at cathode is 0.216 g or approximately 0.22 g.
The correct option is D.
When quantity of electricity passed is one faraday then the mass deposited at the electrode is equal to
- a)One gm. atomic weight
- b)One gm. equivalent weight
- c)Electrochemical equivalent
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
When quantity of electricity passed is one faraday then the mass deposited at the electrode is equal to
a)
One gm. atomic weight
b)
One gm. equivalent weight
c)
Electrochemical equivalent
d)
None of the above
|
|
Upasana Kulkarni answered |
Explanation:
Faraday's laws of electrolysis state that the amount of chemical change produced by a current is proportional to the quantity of electricity passed. The mass of the substance deposited or liberated at the electrode can be calculated using the relation:
Mass deposited = (Current × Time × Equivalent weight) / (96500)
where, Current is the electric current, Time is the duration for which the current flows, Equivalent weight is the atomic weight divided by the valency or the molecular weight divided by the number of electrons involved, and 96500 is the Faraday constant.
When the quantity of electricity passed is one faraday, i.e., 96500 coulombs, the mass deposited at the electrode can be calculated as:
Mass deposited = (96500 × Equivalent weight) / (96500)
Mass deposited = Equivalent weight
Therefore, the correct option is B) One gm. equivalent weight.
Note: The electrochemical equivalent is defined as the mass of a substance deposited or liberated at the electrode per unit quantity of electricity passed, usually expressed in grams per coulomb. It is equal to the equivalent weight divided by 96500.
Faraday's laws of electrolysis state that the amount of chemical change produced by a current is proportional to the quantity of electricity passed. The mass of the substance deposited or liberated at the electrode can be calculated using the relation:
Mass deposited = (Current × Time × Equivalent weight) / (96500)
where, Current is the electric current, Time is the duration for which the current flows, Equivalent weight is the atomic weight divided by the valency or the molecular weight divided by the number of electrons involved, and 96500 is the Faraday constant.
When the quantity of electricity passed is one faraday, i.e., 96500 coulombs, the mass deposited at the electrode can be calculated as:
Mass deposited = (96500 × Equivalent weight) / (96500)
Mass deposited = Equivalent weight
Therefore, the correct option is B) One gm. equivalent weight.
Note: The electrochemical equivalent is defined as the mass of a substance deposited or liberated at the electrode per unit quantity of electricity passed, usually expressed in grams per coulomb. It is equal to the equivalent weight divided by 96500.
1-Butene may be converted to butane by reaction with- a)Zn-HCl
- b)Sn-HCl
- c)Zn-Hg
- d)Pd/H2
Correct answer is option 'D'. Can you explain this answer?
1-Butene may be converted to butane by reaction with
a)
Zn-HCl
b)
Sn-HCl
c)
Zn-Hg
d)
Pd/H2
|
|
Manasa Das answered |
Conversion of 1-Butene to Butane by Pd/H2
1. Introduction:
1-Butene is an unsaturated hydrocarbon with the formula C4H8. It is a linear alkene and is used in the production of many industrial chemicals. Butane, on the other hand, is an alkane with the formula C4H10. It is a saturated hydrocarbon that is commonly used as a fuel. In this question, we are asked to identify the reagent that can be used to convert 1-butene to butane.
2. Explanation:
The correct answer is option 'D', which is Pd/H2. This reaction is known as hydrogenation, which is the addition of hydrogen to an unsaturated compound. In this case, the double bond in 1-butene is converted to a single bond, resulting in the formation of butane.
The reaction mechanism involves the following steps:
- The 1-butene molecule is adsorbed onto the surface of the Pd/H2 catalyst.
- Hydrogen gas (H2) is then adsorbed onto the same surface, adjacent to the adsorbed 1-butene.
- The hydrogen atom on the surface of the Pd/H2 catalyst then migrates to the adsorbed 1-butene molecule, resulting in the formation of a carbon-hydrogen (C-H) bond.
- The other hydrogen atom on the surface of the Pd/H2 catalyst migrates to the adjacent carbon atom, resulting in the formation of another C-H bond.
- The result of these two steps is the addition of two hydrogen atoms to the 1-butene molecule, resulting in the formation of butane.
The overall chemical equation for this reaction is:
1-butene + H2 → butane
3. Conclusion:
In conclusion, the reagent that can be used to convert 1-butene to butane is Pd/H2. This reaction is known as hydrogenation, and it involves the addition of hydrogen to the unsaturated double bond in 1-butene, resulting in the formation of butane.
1. Introduction:
1-Butene is an unsaturated hydrocarbon with the formula C4H8. It is a linear alkene and is used in the production of many industrial chemicals. Butane, on the other hand, is an alkane with the formula C4H10. It is a saturated hydrocarbon that is commonly used as a fuel. In this question, we are asked to identify the reagent that can be used to convert 1-butene to butane.
2. Explanation:
The correct answer is option 'D', which is Pd/H2. This reaction is known as hydrogenation, which is the addition of hydrogen to an unsaturated compound. In this case, the double bond in 1-butene is converted to a single bond, resulting in the formation of butane.
The reaction mechanism involves the following steps:
- The 1-butene molecule is adsorbed onto the surface of the Pd/H2 catalyst.
- Hydrogen gas (H2) is then adsorbed onto the same surface, adjacent to the adsorbed 1-butene.
- The hydrogen atom on the surface of the Pd/H2 catalyst then migrates to the adsorbed 1-butene molecule, resulting in the formation of a carbon-hydrogen (C-H) bond.
- The other hydrogen atom on the surface of the Pd/H2 catalyst migrates to the adjacent carbon atom, resulting in the formation of another C-H bond.
- The result of these two steps is the addition of two hydrogen atoms to the 1-butene molecule, resulting in the formation of butane.
The overall chemical equation for this reaction is:
1-butene + H2 → butane
3. Conclusion:
In conclusion, the reagent that can be used to convert 1-butene to butane is Pd/H2. This reaction is known as hydrogenation, and it involves the addition of hydrogen to the unsaturated double bond in 1-butene, resulting in the formation of butane.
What is hybridization of P in PCl₅?
- a)sp3
- b)sp3d2
- c)sp3d
- d)sp2
Correct answer is option 'C'. Can you explain this answer?
What is hybridization of P in PCl₅?
a)
sp3
b)
sp3d2
c)
sp3d
d)
sp2

|
Manish Aggarwal answered |
The hybridization between P and Cl in PCl5 is sp3d. This is because P in its ground state has the valence electrons occupancy as two in s orbital, three in p orbitals since it has five valence electrons.
A reagent that can separate Fe and Zn is- a)NaOH
- b)HCl
- c)H2S
- d)NaNO2
Correct answer is option 'A'. Can you explain this answer?
A reagent that can separate Fe and Zn is
a)
NaOH
b)
HCl
c)
H2S
d)
NaNO2
|
|
Nitya Sarkar answered |
The correct answer is option 'A' - NaOH.
Explanation:
When Fe and Zn are present together, they cannot be separated by simple physical methods. However, chemical methods can be used to separate them.
One such method is the use of a reagent that can selectively react with one of the metals, leaving the other metal unaffected. NaOH is such a reagent that can selectively react with Fe, leaving Zn unaffected.
The reaction between NaOH and Fe is as follows:
Fe + 2NaOH + 6H2O → 2Na[Fe(OH)4] + 3H2
The reaction between NaOH and Zn is as follows:
Zn + 2NaOH → Na2[Zn(OH)4] + H2
From the above equations, it is clear that NaOH reacts with Fe to form Na[Fe(OH)4], which is soluble in water, and can be separated from Zn by filtration. On the other hand, NaOH reacts with Zn to form Na2[Zn(OH)4], which is insoluble in water, and can be separated from Fe by filtration.
Thus, NaOH can be used to selectively separate Fe and Zn from a mixture of the two metals.
Explanation:
When Fe and Zn are present together, they cannot be separated by simple physical methods. However, chemical methods can be used to separate them.
One such method is the use of a reagent that can selectively react with one of the metals, leaving the other metal unaffected. NaOH is such a reagent that can selectively react with Fe, leaving Zn unaffected.
The reaction between NaOH and Fe is as follows:
Fe + 2NaOH + 6H2O → 2Na[Fe(OH)4] + 3H2
The reaction between NaOH and Zn is as follows:
Zn + 2NaOH → Na2[Zn(OH)4] + H2
From the above equations, it is clear that NaOH reacts with Fe to form Na[Fe(OH)4], which is soluble in water, and can be separated from Zn by filtration. On the other hand, NaOH reacts with Zn to form Na2[Zn(OH)4], which is insoluble in water, and can be separated from Fe by filtration.
Thus, NaOH can be used to selectively separate Fe and Zn from a mixture of the two metals.
2-acetoxy benzoic acid is used as an
- a)antiseptic
- b)antipyretic
- c)antimalarial
- d)antidepressant
Correct answer is option 'B'. Can you explain this answer?
2-acetoxy benzoic acid is used as an
a)
antiseptic
b)
antipyretic
c)
antimalarial
d)
antidepressant

|
Learners Habitat answered |
2- Acetoxybenzoic acid is the chemical name of aspirin. Aspirin is a commonly used antipyretic medicine i.e. medicine to lower the fever.
If in a container, no mass and heat exchange occurs, then it constitutes a/an
- a)open system
- b)isolated system
- c)closed system
- d)imaginary system
Correct answer is option 'B'. Can you explain this answer?
If in a container, no mass and heat exchange occurs, then it constitutes a/an
a)
open system
b)
isolated system
c)
closed system
d)
imaginary system
|
|
Pallavi Ahuja answered |
Closed System in Thermodynamics
A closed system in thermodynamics is a system in which no mass exchange occurs with its surroundings, but energy exchange is allowed. This system is also known as a control mass system.
Explanation
In this question, we are given that there is no mass and heat exchange occurs in a container. This means that the system is not exchanging mass with its surroundings, i.e., it is not an open system. Moreover, it is not an isolated system either because heat exchange is allowed. An isolated system is one in which neither mass nor energy exchange is allowed with its surroundings.
Therefore, the given system is a closed system because it does not exchange mass with its surroundings, but energy exchange is allowed. In a closed system, the energy can be transferred in the form of heat or work, but the mass remains constant.
Conclusion
A closed system is a type of thermodynamic system in which no mass exchange occurs with its surroundings, but energy exchange is allowed. The given system is a closed system because it does not exchange mass with its surroundings, but energy exchange is allowed.
A closed system in thermodynamics is a system in which no mass exchange occurs with its surroundings, but energy exchange is allowed. This system is also known as a control mass system.
Explanation
In this question, we are given that there is no mass and heat exchange occurs in a container. This means that the system is not exchanging mass with its surroundings, i.e., it is not an open system. Moreover, it is not an isolated system either because heat exchange is allowed. An isolated system is one in which neither mass nor energy exchange is allowed with its surroundings.
Therefore, the given system is a closed system because it does not exchange mass with its surroundings, but energy exchange is allowed. In a closed system, the energy can be transferred in the form of heat or work, but the mass remains constant.
Conclusion
A closed system is a type of thermodynamic system in which no mass exchange occurs with its surroundings, but energy exchange is allowed. The given system is a closed system because it does not exchange mass with its surroundings, but energy exchange is allowed.
Ethyl alcohol on oxidation with K₂Cr₂O₇ gives- a) Acetic acid
- b) Acetaldehyde
- c) Formaldehyde
- d) Formic acid
Correct answer is option 'A'. Can you explain this answer?
a)
Acetic acid
b)
Acetaldehyde
c)
Formaldehyde
d)
Formic acid
|
|
Lakshmi Mishra answered |
Oxidation of Ethyl Alcohol with KCrO4 to form Acetic Acid
Oxidation of Ethyl Alcohol with Potassium Dichromate (K2Cr2O7) is a redox reaction. In this reaction, ethyl alcohol is oxidized to acetic acid by the strong oxidizing agent potassium dichromate. The process of oxidizing alcohol is a common reaction in organic chemistry.
The balanced chemical equation for the reaction is given as follows:
3 CH3CH2OH + 2 K2Cr2O7 + 8 H2SO4 → 3 CH3COOH + 2 Cr2(SO4)3 + 2 K2SO4 + 11 H2O
Explanation of the reaction:
- In the reaction, potassium dichromate acts as a strong oxidizing agent.
- Ethyl alcohol is a primary alcohol and it can be converted to an aldehyde or a carboxylic acid by oxidation.
- However, in the presence of strong oxidizing agents like potassium dichromate, the oxidation of alcohol goes all the way to the carboxylic acid stage.
- The oxidation is carried out in the presence of sulfuric acid, which acts as a catalyst.
- The reaction is exothermic and the heat evolved is sufficient to boil the water and sulfuric acid mixture.
- The reaction mixture is then cooled and the acetic acid is purified by distillation.
Conclusion
Hence, option A, i.e., acetic acid is the correct answer.
Oxidation of Ethyl Alcohol with Potassium Dichromate (K2Cr2O7) is a redox reaction. In this reaction, ethyl alcohol is oxidized to acetic acid by the strong oxidizing agent potassium dichromate. The process of oxidizing alcohol is a common reaction in organic chemistry.
The balanced chemical equation for the reaction is given as follows:
3 CH3CH2OH + 2 K2Cr2O7 + 8 H2SO4 → 3 CH3COOH + 2 Cr2(SO4)3 + 2 K2SO4 + 11 H2O
Explanation of the reaction:
- In the reaction, potassium dichromate acts as a strong oxidizing agent.
- Ethyl alcohol is a primary alcohol and it can be converted to an aldehyde or a carboxylic acid by oxidation.
- However, in the presence of strong oxidizing agents like potassium dichromate, the oxidation of alcohol goes all the way to the carboxylic acid stage.
- The oxidation is carried out in the presence of sulfuric acid, which acts as a catalyst.
- The reaction is exothermic and the heat evolved is sufficient to boil the water and sulfuric acid mixture.
- The reaction mixture is then cooled and the acetic acid is purified by distillation.
Conclusion
Hence, option A, i.e., acetic acid is the correct answer.
From a solution of CuSO₄, the metal used to recover copper is- a)Na
- b)Ag
- c)Hg
- d)Fe
Correct answer is option 'D'. Can you explain this answer?
From a solution of CuSO₄, the metal used to recover copper is
a)
Na
b)
Ag
c)
Hg
d)
Fe
|
|
Rishabh Yadav answered |
Recovering Copper from CuSO4 Solution
CuSO4 solution contains copper ions and sulfate ions. To recover copper from this solution, a metal with a higher reduction potential than copper is required. The metal will displace copper ions from the solution and form a solid metal precipitate.
Options:
a) Na (Sodium)
- Sodium has a lower reduction potential than copper.
- Sodium cannot displace copper from the solution.
- Therefore, option 'A' is incorrect.
b) Ag (Silver)
- Silver has a lower reduction potential than copper.
- Silver cannot displace copper from the solution.
- Therefore, option 'B' is incorrect.
c) Hg (Mercury)
- Mercury has a lower reduction potential than copper.
- Mercury cannot displace copper from the solution.
- Therefore, option 'C' is incorrect.
d) Fe (Iron)
- Iron has a higher reduction potential than copper.
- Iron can displace copper from the solution.
- Therefore, option 'D' is correct.
Conclusion:
To recover copper from a CuSO4 solution, a metal with a higher reduction potential than copper is required. Iron is the metal that can displace copper from the solution and form a solid metal precipitate.
CuSO4 solution contains copper ions and sulfate ions. To recover copper from this solution, a metal with a higher reduction potential than copper is required. The metal will displace copper ions from the solution and form a solid metal precipitate.
Options:
a) Na (Sodium)
- Sodium has a lower reduction potential than copper.
- Sodium cannot displace copper from the solution.
- Therefore, option 'A' is incorrect.
b) Ag (Silver)
- Silver has a lower reduction potential than copper.
- Silver cannot displace copper from the solution.
- Therefore, option 'B' is incorrect.
c) Hg (Mercury)
- Mercury has a lower reduction potential than copper.
- Mercury cannot displace copper from the solution.
- Therefore, option 'C' is incorrect.
d) Fe (Iron)
- Iron has a higher reduction potential than copper.
- Iron can displace copper from the solution.
- Therefore, option 'D' is correct.
Conclusion:
To recover copper from a CuSO4 solution, a metal with a higher reduction potential than copper is required. Iron is the metal that can displace copper from the solution and form a solid metal precipitate.
The internal energy of a subtance does not depend upon- a)rotational energy
- b)vibrational energy
- c)translational energy
- d)energy due to gravitational pull
Correct answer is option 'D'. Can you explain this answer?
The internal energy of a subtance does not depend upon
a)
rotational energy
b)
vibrational energy
c)
translational energy
d)
energy due to gravitational pull
|
|
Bhavana Gupta answered |
Internal Energy of a Substance
Internal energy of a substance is the sum of all the energy possessed by its molecules. It includes all forms of energy, such as kinetic energy, potential energy, and chemical energy, that are associated with the molecules of the substance.
Factors that Affect Internal Energy
There are several factors that affect the internal energy of a substance. These include:
1. Temperature: The internal energy of a substance increases with an increase in temperature.
2. Pressure: The internal energy of a substance increases with an increase in pressure.
3. Composition: The internal energy of a substance depends on its chemical composition.
4. Phase: The internal energy of a substance depends on its phase (solid, liquid, or gas).
What does not affect Internal Energy
The internal energy of a substance is independent of certain factors. These include:
1. Rotational Energy: The internal energy of a substance is not affected by the rotational energy of its molecules.
2. Vibrational Energy: The internal energy of a substance is not affected by the vibrational energy of its molecules.
3. Translational Energy: The internal energy of a substance is not affected by the translational energy of its molecules.
4. Energy Due to Gravitational Pull: The internal energy of a substance is not affected by the energy due to gravitational pull. This is because the internal energy is related to the motion and interactions of the molecules within the substance, and not to their position in a gravitational field.
Internal energy of a substance is the sum of all the energy possessed by its molecules. It includes all forms of energy, such as kinetic energy, potential energy, and chemical energy, that are associated with the molecules of the substance.
Factors that Affect Internal Energy
There are several factors that affect the internal energy of a substance. These include:
1. Temperature: The internal energy of a substance increases with an increase in temperature.
2. Pressure: The internal energy of a substance increases with an increase in pressure.
3. Composition: The internal energy of a substance depends on its chemical composition.
4. Phase: The internal energy of a substance depends on its phase (solid, liquid, or gas).
What does not affect Internal Energy
The internal energy of a substance is independent of certain factors. These include:
1. Rotational Energy: The internal energy of a substance is not affected by the rotational energy of its molecules.
2. Vibrational Energy: The internal energy of a substance is not affected by the vibrational energy of its molecules.
3. Translational Energy: The internal energy of a substance is not affected by the translational energy of its molecules.
4. Energy Due to Gravitational Pull: The internal energy of a substance is not affected by the energy due to gravitational pull. This is because the internal energy is related to the motion and interactions of the molecules within the substance, and not to their position in a gravitational field.
The reaction is spontaneous if the cell potential is- a) Positive
- b) Negative
- c) Zero
- d) Infinite
Correct answer is option 'A'. Can you explain this answer?
a)
Positive
b)
Negative
c)
Zero
d)
Infinite
|
|
Avik Saini answered |
Explanation:
The spontaneity of a reaction can be determined by calculating the cell potential. The cell potential (Ecell) is the difference in the electric potential between two electrodes in a galvanic cell. A positive Ecell indicates that the reaction is spontaneous, while a negative Ecell indicates that the reaction is non-spontaneous.
Reasons why a positive Ecell indicates a spontaneous reaction:
1. Gibbs Free Energy (ΔG): The Gibbs free energy (ΔG) of a reaction is a measure of the energy available to do work. A negative ΔG indicates that the reaction is spontaneous, while a positive ΔG indicates that the reaction is non-spontaneous. The relationship between ΔG and Ecell is given by the equation: ΔG = -nFEcell, where n is the number of electrons transferred in the reaction and F is Faraday's constant. Since ΔG is negative for a spontaneous reaction, Ecell must be positive.
2. Thermodynamics: The second law of thermodynamics states that spontaneous processes are those that increase the entropy (disorder) of the system. In an electrochemical cell, the flow of electrons from the anode to the cathode results in an increase in entropy. This flow of electrons occurs spontaneously when Ecell is positive.
3. Electrostatics: The direction of electron flow in an electrochemical cell is determined by the difference in electric potential between the two electrodes. The electrons flow from the anode to the cathode because the cathode has a higher electric potential than the anode. This difference in electric potential results in a positive Ecell, indicating a spontaneous reaction.
In conclusion, a positive Ecell indicates that the reaction is spontaneous because it satisfies the conditions of thermodynamics, electrostatics and Gibbs free energy.
The spontaneity of a reaction can be determined by calculating the cell potential. The cell potential (Ecell) is the difference in the electric potential between two electrodes in a galvanic cell. A positive Ecell indicates that the reaction is spontaneous, while a negative Ecell indicates that the reaction is non-spontaneous.
Reasons why a positive Ecell indicates a spontaneous reaction:
1. Gibbs Free Energy (ΔG): The Gibbs free energy (ΔG) of a reaction is a measure of the energy available to do work. A negative ΔG indicates that the reaction is spontaneous, while a positive ΔG indicates that the reaction is non-spontaneous. The relationship between ΔG and Ecell is given by the equation: ΔG = -nFEcell, where n is the number of electrons transferred in the reaction and F is Faraday's constant. Since ΔG is negative for a spontaneous reaction, Ecell must be positive.
2. Thermodynamics: The second law of thermodynamics states that spontaneous processes are those that increase the entropy (disorder) of the system. In an electrochemical cell, the flow of electrons from the anode to the cathode results in an increase in entropy. This flow of electrons occurs spontaneously when Ecell is positive.
3. Electrostatics: The direction of electron flow in an electrochemical cell is determined by the difference in electric potential between the two electrodes. The electrons flow from the anode to the cathode because the cathode has a higher electric potential than the anode. This difference in electric potential results in a positive Ecell, indicating a spontaneous reaction.
In conclusion, a positive Ecell indicates that the reaction is spontaneous because it satisfies the conditions of thermodynamics, electrostatics and Gibbs free energy.
[ Ni(CN)4 ]2− , [ MnBr4 ]2− and [ FeF6]3−have geometry, hybridization and magnetic moments of the ions respectively- a)Tetrahedral, square planar, octahedral : sp3, dsp2,sp3d2; 5.9,0,4.9
- b)Tetrahedral, square planar, octahedral : dsp2, sp3,sp3d2; 0,5.9,4.9
- c)Square planar,tetrahedral, octahedral : dsp2, sp3,d2sp3; 5.9,4.9,0
- d)Square planar,tetrahedral, octahedral : dsp2, sp3,sp3d2}; 0,5.9,4.9
Correct answer is option 'D'. Can you explain this answer?
[ Ni(CN)4 ]2− , [ MnBr4 ]2− and [ FeF6]3−
have geometry, hybridization and magnetic moments of the ions respectively
a)
Tetrahedral, square planar, octahedral : sp3, dsp2,sp3d2; 5.9,0,4.9
b)
Tetrahedral, square planar, octahedral : dsp2, sp3,sp3d2; 0,5.9,4.9
c)
Square planar,tetrahedral, octahedral : dsp2, sp3,d2sp3; 5.9,4.9,0
d)
Square planar,tetrahedral, octahedral : dsp2, sp3,sp3d2}; 0,5.9,4.9
|
|
Abhiram Sen answered |
The name of the compound [ Ni(CN)4 ]2 is nickel(II) tetracyanide.
At which one of the following temperature-pressure conditions, the deviation of a gas from ideal behaviour is expected to be minimum ?- a)550 K and 1 atm
- b)350 K and 3 atm
- c)250 K and 4 atm
- d)450 K and 2 atm
Correct answer is option 'A'. Can you explain this answer?
At which one of the following temperature-pressure conditions, the deviation of a gas from ideal behaviour is expected to be minimum ?
a)
550 K and 1 atm
b)
350 K and 3 atm
c)
250 K and 4 atm
d)
450 K and 2 atm
|
|
Dishani Sen answered |
Ideal Gas Law and Deviation from Ideal Behaviour
The ideal gas law is a fundamental concept in thermodynamics that describes the behaviour of gases. According to the ideal gas law, the pressure, volume and temperature of a gas are related by the equation PV=nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature. This equation assumes that the gas behaves as an ideal gas, which means that the gas molecules are infinitely small, do not interact with each other, and have no internal energy.
However, in reality, gases do not always behave as ideal gases, especially at high pressures and low temperatures. At these conditions, the gas molecules are no longer infinitely small and start to interact with each other, which leads to deviations from ideal behaviour.
Factors Affecting Deviation from Ideal Behaviour
The deviation of a gas from ideal behaviour depends on several factors, including temperature, pressure, and the nature of the gas molecules. At high temperatures and low pressures, the gas molecules have high kinetic energy and move freely, which reduces their interactions with each other. At low temperatures and high pressures, the gas molecules are closer together and interact more strongly, which increases the deviation from ideal behaviour.
Answer Explanation
Among the given options, option 'A' has the highest temperature and the lowest pressure, which means that the gas molecules have high kinetic energy and move freely, while their interactions with each other are minimal. Therefore, at this condition, the deviation of the gas from ideal behaviour is expected to be minimum. Hence, the correct answer is option 'A'.
Conclusion
The deviation of a gas from ideal behaviour depends on several factors, including temperature, pressure, and the nature of the gas molecules. At high temperatures and low pressures, the gas molecules have high kinetic energy and move freely, which reduces their interactions with each other. Therefore, the deviation of the gas from ideal behaviour is expected to be minimum at this condition.
The ideal gas law is a fundamental concept in thermodynamics that describes the behaviour of gases. According to the ideal gas law, the pressure, volume and temperature of a gas are related by the equation PV=nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature. This equation assumes that the gas behaves as an ideal gas, which means that the gas molecules are infinitely small, do not interact with each other, and have no internal energy.
However, in reality, gases do not always behave as ideal gases, especially at high pressures and low temperatures. At these conditions, the gas molecules are no longer infinitely small and start to interact with each other, which leads to deviations from ideal behaviour.
Factors Affecting Deviation from Ideal Behaviour
The deviation of a gas from ideal behaviour depends on several factors, including temperature, pressure, and the nature of the gas molecules. At high temperatures and low pressures, the gas molecules have high kinetic energy and move freely, which reduces their interactions with each other. At low temperatures and high pressures, the gas molecules are closer together and interact more strongly, which increases the deviation from ideal behaviour.
Answer Explanation
Among the given options, option 'A' has the highest temperature and the lowest pressure, which means that the gas molecules have high kinetic energy and move freely, while their interactions with each other are minimal. Therefore, at this condition, the deviation of the gas from ideal behaviour is expected to be minimum. Hence, the correct answer is option 'A'.
Conclusion
The deviation of a gas from ideal behaviour depends on several factors, including temperature, pressure, and the nature of the gas molecules. At high temperatures and low pressures, the gas molecules have high kinetic energy and move freely, which reduces their interactions with each other. Therefore, the deviation of the gas from ideal behaviour is expected to be minimum at this condition.
Which of the following compound gives dye test ?
- a)Aniline
- b)Methylamine
- c)Diphenyl amine
- d)Ethylamine
Correct answer is option 'A'. Can you explain this answer?
Which of the following compound gives dye test ?
a)
Aniline
b)
Methylamine
c)
Diphenyl amine
d)
Ethylamine
|
|
Tejas Verma answered |
Dye test is given by Aromatic 1∘ amines
A cylinder filled with a movable piston contains liquid water in equilibrium with water vapour at 25oC. Which one of the following operations results in a decreace in the equilibrium vapour pressure ?- a)Moving the piston downward a short distance
- b)Removing a small amount of vapour
- c)Removing a small amount of the liquid water
- d)Dissolving salt in the water
Correct answer is option 'D'. Can you explain this answer?
A cylinder filled with a movable piston contains liquid water in equilibrium with water vapour at 25oC. Which one of the following operations results in a decreace in the equilibrium vapour pressure ?
a)
Moving the piston downward a short distance
b)
Removing a small amount of vapour
c)
Removing a small amount of the liquid water
d)
Dissolving salt in the water
|
|
Genius answered |
Obviously, because vapour of solution is less than the vapour pressure of pure solvent.
0.066 gram of metal was deposited when a current of 2 amperes is passed through a metal ion solution for 100 seconds. What is the electrochemical equivalent (in gram coulomb -1) of the metal?- a)3. 3 x 10−6
- b)3.3 x 10−4
- c)0.033
- d)3.3
Correct answer is option 'B'. Can you explain this answer?
0.066 gram of metal was deposited when a current of 2 amperes is passed through a metal ion solution for 100 seconds. What is the electrochemical equivalent (in gram coulomb -1) of the metal?
a)
3. 3 x 10−6
b)
3.3 x 10−4
c)
0.033
d)
3.3
|
|
Rohit Jain answered |
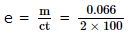
= 3.3 x 10-4 gm coulomb
Which of the following reagent react differently with HCHO and CH₃CHO and CH₃COCH₃- a)HCN
- b)NH₂-NH₂
- c)NH₂OH
- d)NH₃
Correct answer is option 'D'. Can you explain this answer?
Which of the following reagent react differently with HCHO and CH₃CHO and CH₃COCH₃
a)
HCN
b)
NH₂-NH₂
c)
NH₂OH
d)
NH₃
|
|
Simran Chopra answered |
Reactivity of reagents with different aldehydes and ketones:
Aldehydes and ketones have a carbonyl group (C=O) which makes them highly reactive towards nucleophiles. However, different aldehydes and ketones have different reactivity towards different nucleophiles. Let's take a look at the reactivity of the given reagents with different aldehydes and ketones.
a) HCN (Hydrogen Cyanide):
- Reacts with aldehydes and ketones to form cyanohydrins.
- The reaction is nucleophilic addition of HCN to the carbonyl group.
- The reaction is reversible and the equilibrium lies towards the formation of the cyanohydrin.
b) NH2NH2 (Hydrazine):
- Reacts with ketones to form hydrazones.
- The reaction is nucleophilic addition of the hydrazine to the carbonyl group followed by elimination of water.
- The reaction is irreversible and the product is stable.
c) NHOH (Hydroxylamine):
- Reacts with ketones to form oximes.
- The reaction is nucleophilic addition of the hydroxylamine to the carbonyl group followed by elimination of water.
- The reaction is irreversible and the product is stable.
d) NH3 (Ammonia):
- Reacts with aldehydes and ketones to form imines.
- The reaction is nucleophilic addition of the ammonia to the carbonyl group followed by elimination of water.
- The reaction is reversible and the equilibrium lies towards the formation of the imine.
Reactivity of HCHO, CH3CHO, and CH3COCH3 with NH3:
HCHO (Formaldehyde):
- Reacts with NH3 to form imine.
- The reaction is reversible and the equilibrium lies towards the formation of the imine.
CH3CHO (Acetaldehyde):
- Reacts with NH3 to form imine.
- The reaction is reversible and the equilibrium lies towards the formation of the imine.
CH3COCH3 (Acetone):
- Reacts with NH3 to form enamine.
- The reaction is reversible and the equilibrium lies towards the formation of the enamine.
Therefore, the correct answer is option D, NH3, as it reacts differently with CH3COCH3 compared to HCHO and CH3CHO.
Aldehydes and ketones have a carbonyl group (C=O) which makes them highly reactive towards nucleophiles. However, different aldehydes and ketones have different reactivity towards different nucleophiles. Let's take a look at the reactivity of the given reagents with different aldehydes and ketones.
a) HCN (Hydrogen Cyanide):
- Reacts with aldehydes and ketones to form cyanohydrins.
- The reaction is nucleophilic addition of HCN to the carbonyl group.
- The reaction is reversible and the equilibrium lies towards the formation of the cyanohydrin.
b) NH2NH2 (Hydrazine):
- Reacts with ketones to form hydrazones.
- The reaction is nucleophilic addition of the hydrazine to the carbonyl group followed by elimination of water.
- The reaction is irreversible and the product is stable.
c) NHOH (Hydroxylamine):
- Reacts with ketones to form oximes.
- The reaction is nucleophilic addition of the hydroxylamine to the carbonyl group followed by elimination of water.
- The reaction is irreversible and the product is stable.
d) NH3 (Ammonia):
- Reacts with aldehydes and ketones to form imines.
- The reaction is nucleophilic addition of the ammonia to the carbonyl group followed by elimination of water.
- The reaction is reversible and the equilibrium lies towards the formation of the imine.
Reactivity of HCHO, CH3CHO, and CH3COCH3 with NH3:
HCHO (Formaldehyde):
- Reacts with NH3 to form imine.
- The reaction is reversible and the equilibrium lies towards the formation of the imine.
CH3CHO (Acetaldehyde):
- Reacts with NH3 to form imine.
- The reaction is reversible and the equilibrium lies towards the formation of the imine.
CH3COCH3 (Acetone):
- Reacts with NH3 to form enamine.
- The reaction is reversible and the equilibrium lies towards the formation of the enamine.
Therefore, the correct answer is option D, NH3, as it reacts differently with CH3COCH3 compared to HCHO and CH3CHO.
Which is correct statement?
- a)Starch is a polymer of β -glucose
- b)Amylose is a component of cellulose
- c)Proteins are compounds of only one type of amino acid
- d)In cyclic structure of frucstose, there are four carbons and one oxygen atom
Correct answer is option 'D'. Can you explain this answer?
Which is correct statement?
a)
Starch is a polymer of β -glucose
b)
Amylose is a component of cellulose
c)
Proteins are compounds of only one type of amino acid
d)
In cyclic structure of frucstose, there are four carbons and one oxygen atom
|
|
Nidhi Sen answered |
Glucose
b)Glucose is a polymer of starch
The correct statement is a) Starch is a polymer of glucose.
b)Glucose is a polymer of starch
The correct statement is a) Starch is a polymer of glucose.
P4O10 has short and long P-O bonds. The number of short P-O bonds in this compound is- a)1
- b)3
- c)2
- d)4
Correct answer is option 'D'. Can you explain this answer?
P4O10 has short and long P-O bonds. The number of short P-O bonds in this compound is
a)
1
b)
3
c)
2
d)
4
|
|
Nabanita Rane answered |
P4O10 has four P = O bonds which are shorter than P-O single bonds.
Each P atom has three P-O single bonds and one P = O bond, i.e.,
a total of four P-O linkages
Each P atom has three P-O single bonds and one P = O bond, i.e.,
a total of four P-O linkages
The reaction, CH₂ = CH-CH₃ + HBr → CH₃CHBr - CH₃ is- a)Nucleophilic addition
- b)Electrophilic substitution
- c)Electrophilic addition
- d)Free radical addition
Correct answer is option 'C'. Can you explain this answer?
The reaction, CH₂ = CH-CH₃ + HBr → CH₃CHBr - CH₃ is
a)
Nucleophilic addition
b)
Electrophilic substitution
c)
Electrophilic addition
d)
Free radical addition
|
|
Uday Ghoshal answered |
Electrophilic Addition
This reaction involves the addition of an electrophile (HBr) to a double bond (CH=CH-CH). The double bond acts as a nucleophile, attacking the electrophile to form a new bond. This type of reaction is known as electrophilic addition.
Mechanism:
1. The double bond in CH=CH-CH acts as a nucleophile and attacks the electrophile HBr.
2. The pi bond breaks as the nucleophile attacks, forming a carbocation intermediate.
3. The Br- ion then attacks the carbocation, forming the final product CHCHBr-CH.
Characteristics of Electrophilic Addition:
- Involves the addition of an electrophile to a double bond.
- Forms a carbocation intermediate.
- Typically occurs with alkenes or alkynes.
- Product is formed by addition of electrophile to the double bond.
In this reaction, the electrophile HBr adds to the double bond in CH=CH-CH, resulting in the formation of CHCHBr-CH. This reaction follows the characteristics of electrophilic addition, making option 'C' the correct answer.
This reaction involves the addition of an electrophile (HBr) to a double bond (CH=CH-CH). The double bond acts as a nucleophile, attacking the electrophile to form a new bond. This type of reaction is known as electrophilic addition.
Mechanism:
1. The double bond in CH=CH-CH acts as a nucleophile and attacks the electrophile HBr.
2. The pi bond breaks as the nucleophile attacks, forming a carbocation intermediate.
3. The Br- ion then attacks the carbocation, forming the final product CHCHBr-CH.
Characteristics of Electrophilic Addition:
- Involves the addition of an electrophile to a double bond.
- Forms a carbocation intermediate.
- Typically occurs with alkenes or alkynes.
- Product is formed by addition of electrophile to the double bond.
In this reaction, the electrophile HBr adds to the double bond in CH=CH-CH, resulting in the formation of CHCHBr-CH. This reaction follows the characteristics of electrophilic addition, making option 'C' the correct answer.
When H2 and I2 are mixed and equilibrium is attained,then- a)amount of HI formed is equal to the amount of H2 dissociated
- b)HI dissociated stops
- c)The reaction stops completely
- d)None of these
Correct answer is option 'D'. Can you explain this answer?
When H2 and I2 are mixed and equilibrium is attained,then
a)
amount of HI formed is equal to the amount of H2 dissociated
b)
HI dissociated stops
c)
The reaction stops completely
d)
None of these
|
|
Priya Mishra answered |
At equilibrium rate of forward reaction becomes equal to the rate of backward reaction
In which of the following neutralization reaction, the heat of neutralisation will be highest?- a)NH₄OH and H₂SO₄
- b)HCl and NaOH
- c)CH₃COOH and KOH
- d)H₂C₂O₄ + KOH
Correct answer is option 'B'. Can you explain this answer?
In which of the following neutralization reaction, the heat of neutralisation will be highest?
a)
NH₄OH and H₂SO₄
b)
HCl and NaOH
c)
CH₃COOH and KOH
d)
H₂C₂O₄ + KOH
|
|
Ankita Nambiar answered |
Heat of Neutralisation:
The heat of neutralisation is defined as the amount of heat evolved when one equivalent of an acid and one equivalent of a base react to form a neutral salt in a dilute solution.
Explanation:
To determine which of the given neutralization reactions has the highest heat of neutralisation, we need to consider the strength of the acid and base involved in each reaction.
- Option A: NHOH and HSO - NH4OH is a weak base and H2SO4 is a strong acid. The heat of neutralisation for this reaction will be less than that of other reactions.
- Option B: HCl and NaOH - HCl is a strong acid and NaOH is a strong base. The heat of neutralisation for this reaction will be the highest among all the given options.
- Option C: CH3COOH and KOH - CH3COOH is a weak acid and KOH is a strong base. The heat of neutralisation for this reaction will be less than that of option B.
- Option D: HCOOH and KOH - HCOOH is a weak acid and KOH is a strong base. The heat of neutralisation for this reaction will be less than that of option B.
Therefore, the correct answer is option B - HCl and NaOH, as it involves the reaction between strong acid and strong base, resulting in the highest heat of neutralisation.
The heat of neutralisation is defined as the amount of heat evolved when one equivalent of an acid and one equivalent of a base react to form a neutral salt in a dilute solution.
Explanation:
To determine which of the given neutralization reactions has the highest heat of neutralisation, we need to consider the strength of the acid and base involved in each reaction.
- Option A: NHOH and HSO - NH4OH is a weak base and H2SO4 is a strong acid. The heat of neutralisation for this reaction will be less than that of other reactions.
- Option B: HCl and NaOH - HCl is a strong acid and NaOH is a strong base. The heat of neutralisation for this reaction will be the highest among all the given options.
- Option C: CH3COOH and KOH - CH3COOH is a weak acid and KOH is a strong base. The heat of neutralisation for this reaction will be less than that of option B.
- Option D: HCOOH and KOH - HCOOH is a weak acid and KOH is a strong base. The heat of neutralisation for this reaction will be less than that of option B.
Therefore, the correct answer is option B - HCl and NaOH, as it involves the reaction between strong acid and strong base, resulting in the highest heat of neutralisation.
The protein which maintains blood sugar level is- a)Haemoglobin
- b)Oxytocin
- c)Insulin
- d)Ptyalin
Correct answer is option 'C'. Can you explain this answer?
The protein which maintains blood sugar level is
a)
Haemoglobin
b)
Oxytocin
c)
Insulin
d)
Ptyalin
|
|
Kajal Ahuja answered |
Protein that maintains blood sugar level is Insulin.
Insulin is a protein hormone that is produced and secreted by the pancreas. It plays a crucial role in regulating blood sugar levels by facilitating the uptake of glucose from the blood into cells for energy or storage.
Insulin works by binding to specific receptors on the surface of cells, particularly in the liver, muscle, and fat tissues. This binding triggers a series of biochemical events that result in the transport of glucose from the blood into the cells.
When blood sugar levels rise, the pancreas secretes insulin into the bloodstream. This signals the liver to store excess glucose as glycogen, and it stimulates muscle and fat cells to take up glucose from the blood.
In people with diabetes, the body either does not produce enough insulin or cannot use it effectively, leading to high blood sugar levels. Treatment for diabetes typically involves insulin therapy to help regulate blood sugar levels.
In conclusion, insulin is the protein that maintains blood sugar level by regulating glucose uptake from the blood into cells.
Insulin is a protein hormone that is produced and secreted by the pancreas. It plays a crucial role in regulating blood sugar levels by facilitating the uptake of glucose from the blood into cells for energy or storage.
Insulin works by binding to specific receptors on the surface of cells, particularly in the liver, muscle, and fat tissues. This binding triggers a series of biochemical events that result in the transport of glucose from the blood into the cells.
When blood sugar levels rise, the pancreas secretes insulin into the bloodstream. This signals the liver to store excess glucose as glycogen, and it stimulates muscle and fat cells to take up glucose from the blood.
In people with diabetes, the body either does not produce enough insulin or cannot use it effectively, leading to high blood sugar levels. Treatment for diabetes typically involves insulin therapy to help regulate blood sugar levels.
In conclusion, insulin is the protein that maintains blood sugar level by regulating glucose uptake from the blood into cells.
Which of the following simple ions will not form complex ion with NH₃?- a)Pb2+
- b)Ag2+
- c)Cd2+
- d)Cu2+
Correct answer is option 'A'. Can you explain this answer?
Which of the following simple ions will not form complex ion with NH₃?
a)
Pb2+
b)
Ag2+
c)
Cd2+
d)
Cu2+
|
|
Mrinalini Mukherjee answered |
Explanation:
Complex ion formation is the process of a simple ion combining with one or more ligands (molecules or ions) to form a complex ion. In this process, the simple ion acts as a Lewis acid (electron pair acceptor) and the ligand acts as a Lewis base (electron pair donor).
NH3 is a commonly used ligand in complex ion formation reactions. It has a lone pair of electrons on the nitrogen atom, which can be donated to form a coordinate covalent bond with a metal ion or other cation.
In this question, we are asked to identify the simple ions that will not form a complex ion with NH3. Let's consider each of the options given:
a) Pb2+
Lead(II) ion has a filled d-orbital and is not a good Lewis acid. It also has a high charge density due to its small size, which makes it difficult to accommodate the bulky NH3 ligand. Therefore, Pb2+ is unlikely to form a stable complex ion with NH3.
b) Ag2+
Silver(I) ion has a partially filled d-orbital and is a good Lewis acid. It can form stable complex ions with NH3, such as [Ag(NH3)2]+. Therefore, Ag2+ is likely to form a complex ion with NH3.
c) Cd2+
Cadmium(II) ion has a partially filled d-orbital and is a good Lewis acid. It can form stable complex ions with NH3, such as [Cd(NH3)4]2+. Therefore, Cd2+ is likely to form a complex ion with NH3.
d) Cu2+
Copper(II) ion has a partially filled d-orbital and is a good Lewis acid. It can form stable complex ions with NH3, such as [Cu(NH3)4]2+. Therefore, Cu2+ is likely to form a complex ion with NH3.
Conclusion:
Based on the above analysis, we can conclude that Pb2+ is the simple ion that will not form a complex ion with NH3. Therefore, the correct answer is option 'A'.
Complex ion formation is the process of a simple ion combining with one or more ligands (molecules or ions) to form a complex ion. In this process, the simple ion acts as a Lewis acid (electron pair acceptor) and the ligand acts as a Lewis base (electron pair donor).
NH3 is a commonly used ligand in complex ion formation reactions. It has a lone pair of electrons on the nitrogen atom, which can be donated to form a coordinate covalent bond with a metal ion or other cation.
In this question, we are asked to identify the simple ions that will not form a complex ion with NH3. Let's consider each of the options given:
a) Pb2+
Lead(II) ion has a filled d-orbital and is not a good Lewis acid. It also has a high charge density due to its small size, which makes it difficult to accommodate the bulky NH3 ligand. Therefore, Pb2+ is unlikely to form a stable complex ion with NH3.
b) Ag2+
Silver(I) ion has a partially filled d-orbital and is a good Lewis acid. It can form stable complex ions with NH3, such as [Ag(NH3)2]+. Therefore, Ag2+ is likely to form a complex ion with NH3.
c) Cd2+
Cadmium(II) ion has a partially filled d-orbital and is a good Lewis acid. It can form stable complex ions with NH3, such as [Cd(NH3)4]2+. Therefore, Cd2+ is likely to form a complex ion with NH3.
d) Cu2+
Copper(II) ion has a partially filled d-orbital and is a good Lewis acid. It can form stable complex ions with NH3, such as [Cu(NH3)4]2+. Therefore, Cu2+ is likely to form a complex ion with NH3.
Conclusion:
Based on the above analysis, we can conclude that Pb2+ is the simple ion that will not form a complex ion with NH3. Therefore, the correct answer is option 'A'.
An important ore of copper is- a)Bauxite
- b)Galena
- c)Siderite
- d)Chalcopyrites
Correct answer is option 'D'. Can you explain this answer?
An important ore of copper is
a)
Bauxite
b)
Galena
c)
Siderite
d)
Chalcopyrites

|
Yedhu Nair 94 Nair 94 answered |
Chalcopyrite formula is CuFeS2 from ore you get metals it's a theoritical question you need to know the different ores of different metals
The ether that undergoes electrophilic substitution reaction is- a)CH₃OC₂H₅
- b)C₆H₅OCH₃
- c)CH₃OCH₃
- d)C₂H₅OC₂H₅
Correct answer is option 'B'. Can you explain this answer?
The ether that undergoes electrophilic substitution reaction is
a)
CH₃OC₂H₅
b)
C₆H₅OCH₃
c)
CH₃OCH₃
d)
C₂H₅OC₂H₅
|
|
Shreya Sengupta answered |
Electrophilic substitution reactions involve the attack of an electrophile on an aromatic ring. In order for a compound to undergo electrophilic substitution, it must have a pi electron system that can act as a nucleophile and react with the electrophile.
Among the given options, only option 'B' contains an aromatic ring with a pi electron system, which is capable of undergoing electrophilic substitution. Let's take a closer look at the structure of option 'B'.
The compound in option 'B' is benzaldehyde, which has the following structure:

As we can see, benzaldehyde contains an aromatic ring with a pi electron system, which makes it capable of undergoing electrophilic substitution. The benzaldehyde molecule can react with an electrophile such as a halogen or a nitro group, forming a substituted product.
Therefore, the correct answer is option 'B'.
Among the given options, only option 'B' contains an aromatic ring with a pi electron system, which is capable of undergoing electrophilic substitution. Let's take a closer look at the structure of option 'B'.
The compound in option 'B' is benzaldehyde, which has the following structure:

As we can see, benzaldehyde contains an aromatic ring with a pi electron system, which makes it capable of undergoing electrophilic substitution. The benzaldehyde molecule can react with an electrophile such as a halogen or a nitro group, forming a substituted product.
Therefore, the correct answer is option 'B'.
The e.m.f. of a galvanic cell with electrode potentials of Al equal to -1.66 V and that of Mg equal to - 0.54 V is- a)+ 0.44 V
- b)+ 1.12 V
- c)- 0.44 V
- d)- 1.12 V
Correct answer is option 'D'. Can you explain this answer?
The e.m.f. of a galvanic cell with electrode potentials of Al equal to -1.66 V and that of Mg equal to - 0.54 V is
a)
+ 0.44 V
b)
+ 1.12 V
c)
- 0.44 V
d)
- 1.12 V
|
|
Raji Ram answered |
Electrode potential Mg is greater than EP of Al .
Hence Al is anode and Mg is cathode.
emf = E(cathode) - E(anode) = -0.54- (-1.66) = 1.12V
option B is correct not D
if emf is - ve the reaction is not feasible
When H2 and I2 are mixed and equilibrium is attained,then- a)amount of HI formed is equal to the amount of H2 dissociated
- b)HI dissociated stops
- c)The reaction stops completely
- d)None of these
Correct answer is option 'D'. Can you explain this answer?
When H2 and I2 are mixed and equilibrium is attained,then
a)
amount of HI formed is equal to the amount of H2 dissociated
b)
HI dissociated stops
c)
The reaction stops completely
d)
None of these
|
|
Nitya Basak answered |
At equilibrium rate of forward reaction becomes equal to the rate of backward reaction
In the silver plating of copper, K[Ag(CN)₂] is used insead of AgNO₃. The reason is- a)A thin layer of Ag is formed on Cu
- b)More voltage is required
- c)Ag⁺ ions are completely removed from solution
- d)Less availablity of Ag⁺ ions, as Cu cannot displace Ag from [Ag(CN)₂]⁻ ion.
Correct answer is option 'D'. Can you explain this answer?
In the silver plating of copper, K[Ag(CN)₂] is used insead of AgNO₃. The reason is
a)
A thin layer of Ag is formed on Cu
b)
More voltage is required
c)
Ag⁺ ions are completely removed from solution
d)
Less availablity of Ag⁺ ions, as Cu cannot displace Ag from [Ag(CN)₂]⁻ ion.
|
|
Sagar Yadav answered |
Explanation:
In silver plating, a thin layer of silver is deposited on the surface of copper to enhance its appearance, corrosion resistance, and conductivity. This process involves the use of an electrolytic solution containing silver ions, which are reduced and deposited on the cathode (copper) surface.
However, the choice of silver source in the electrolytic solution can affect the efficiency and quality of the plating process. In the case of K[Ag(CN)], this compound is preferred over AgNO3 for the following reasons:
Less availability of Ag ions:
Cu cannot displace Ag from [Ag(CN)] ion.
AgNO3 is a readily available source of silver ions in solution, but it has a disadvantage that Cu can displace Ag from the solution, leading to the formation of Cu(NO3)2 and depletion of Ag ions. This reduces the efficiency of the plating process and may result in a non-uniform or incomplete coating.
On the other hand, K[Ag(CN)] is a complex compound that contains Ag ions in a more stable form. The cyanide ligands protect the Ag ions from being displaced by Cu ions, as the Cu-CN bond is weaker than the Ag-CN bond. This ensures a steady supply of Ag ions in solution for the plating process.
Moreover, K[Ag(CN)] has a higher solubility and conductivity than AgNO3, which improves the current efficiency and reduces the energy consumption in the plating process.
Conclusion:
Therefore, the use of K[Ag(CN)] in silver plating of copper is a better option than AgNO3, as it provides a stable and efficient source of silver ions for deposition on the copper surface.
In silver plating, a thin layer of silver is deposited on the surface of copper to enhance its appearance, corrosion resistance, and conductivity. This process involves the use of an electrolytic solution containing silver ions, which are reduced and deposited on the cathode (copper) surface.
However, the choice of silver source in the electrolytic solution can affect the efficiency and quality of the plating process. In the case of K[Ag(CN)], this compound is preferred over AgNO3 for the following reasons:
Less availability of Ag ions:
Cu cannot displace Ag from [Ag(CN)] ion.
AgNO3 is a readily available source of silver ions in solution, but it has a disadvantage that Cu can displace Ag from the solution, leading to the formation of Cu(NO3)2 and depletion of Ag ions. This reduces the efficiency of the plating process and may result in a non-uniform or incomplete coating.
On the other hand, K[Ag(CN)] is a complex compound that contains Ag ions in a more stable form. The cyanide ligands protect the Ag ions from being displaced by Cu ions, as the Cu-CN bond is weaker than the Ag-CN bond. This ensures a steady supply of Ag ions in solution for the plating process.
Moreover, K[Ag(CN)] has a higher solubility and conductivity than AgNO3, which improves the current efficiency and reduces the energy consumption in the plating process.
Conclusion:
Therefore, the use of K[Ag(CN)] in silver plating of copper is a better option than AgNO3, as it provides a stable and efficient source of silver ions for deposition on the copper surface.
What happens when conc. H₂SO₄ is treated with sugar?- a)Oxidation
- b)Reduction
- c)Dehydration
- d)Hydrolysis
Correct answer is option 'C'. Can you explain this answer?
What happens when conc. H₂SO₄ is treated with sugar?
a)
Oxidation
b)
Reduction
c)
Dehydration
d)
Hydrolysis
|
|
Avik Nair answered |
Dehydration of Sugar with Conc. HSO:
When concentrated sulfuric acid (HSO) is added to sugar, it undergoes dehydration reaction, resulting in the formation of a black, porous carbon material.
Explanation:
• Sugar is a carbohydrate, and it contains hydroxyl (-OH) groups, which can undergo a dehydration reaction in the presence of concentrated sulfuric acid (HSO).
• The sulfuric acid acts as a dehydrating agent, removing water molecules from the sugar molecule.
• During this process, the hydroxyl groups (-OH) of the sugar molecule are removed as a water molecule (H2O), leaving behind a carbon skeleton.
• The carbon skeleton is highly porous and black in color, giving the reaction mixture a black color.
• This reaction is an example of a dehydration reaction, which involves the removal of water molecules from a molecule to form a new compound.
• The dehydration of sugar with concentrated sulfuric acid is an exothermic reaction, which means that it releases heat.
• The carbon material produced during the reaction is often used as a decolorizing agent in the sugar industry.
• It can also be used as a fuel, although the high porosity of the carbon material makes it difficult to handle and store.
Conclusion:
Thus, when concentrated sulfuric acid is treated with sugar, it undergoes a dehydration reaction, resulting in the formation of a black, porous carbon material.
When concentrated sulfuric acid (HSO) is added to sugar, it undergoes dehydration reaction, resulting in the formation of a black, porous carbon material.
Explanation:
• Sugar is a carbohydrate, and it contains hydroxyl (-OH) groups, which can undergo a dehydration reaction in the presence of concentrated sulfuric acid (HSO).
• The sulfuric acid acts as a dehydrating agent, removing water molecules from the sugar molecule.
• During this process, the hydroxyl groups (-OH) of the sugar molecule are removed as a water molecule (H2O), leaving behind a carbon skeleton.
• The carbon skeleton is highly porous and black in color, giving the reaction mixture a black color.
• This reaction is an example of a dehydration reaction, which involves the removal of water molecules from a molecule to form a new compound.
• The dehydration of sugar with concentrated sulfuric acid is an exothermic reaction, which means that it releases heat.
• The carbon material produced during the reaction is often used as a decolorizing agent in the sugar industry.
• It can also be used as a fuel, although the high porosity of the carbon material makes it difficult to handle and store.
Conclusion:
Thus, when concentrated sulfuric acid is treated with sugar, it undergoes a dehydration reaction, resulting in the formation of a black, porous carbon material.
The quanitity of electricity required to librate 112 cm3 of hydrogen at STP from acidified water is- a)965 C
- b)1 Faraday
- c)0.1 F
- d)96500 C
Correct answer is option 'A'. Can you explain this answer?
The quanitity of electricity required to librate 112 cm3 of hydrogen at STP from acidified water is
a)
965 C
b)
1 Faraday
c)
0.1 F
d)
96500 C
|
|
Deepika Chavan answered |
2H+ + 2e- → H2
2e- → 2F
H2 → 22.4 L at STP
To liberate 112 cm3 at STP electron required
= (2 x 96500 x 112)/ 22400 = 965 C
2e- → 2F
H2 → 22.4 L at STP
To liberate 112 cm3 at STP electron required
= (2 x 96500 x 112)/ 22400 = 965 C
Which of the following does not represent a type of crystal system- a)Triclinic
- b)Monoclinic
- c)Rhombohedral
- d)Isotropical
Correct answer is option 'D'. Can you explain this answer?
Which of the following does not represent a type of crystal system
a)
Triclinic
b)
Monoclinic
c)
Rhombohedral
d)
Isotropical
|
|
Chirag Chakraborty answered |
Isotropical is not a type of crystal system
Crystal systems are a way of categorizing crystals based on their symmetry and geometric properties. Isotropical is not a valid crystal system because it does not exist in the classification of crystal systems.
Types of crystal systems:
- Triclinic: This crystal system has three axes of different lengths that are all inclined relative to each other.
- Monoclinic: This crystal system has three axes of different lengths, with one axis perpendicular to the other two.
- Rhombohedral: This crystal system has three axes of equal length, all intersecting at angles that are not right angles.
Explanation:
Isotropical is not a valid crystal system because it does not describe a specific arrangement of axes and angles that characterize a crystal structure. It is important to understand the different types of crystal systems in order to study the properties and behavior of crystals in materials science and mineralogy.
Crystal systems are a way of categorizing crystals based on their symmetry and geometric properties. Isotropical is not a valid crystal system because it does not exist in the classification of crystal systems.
Types of crystal systems:
- Triclinic: This crystal system has three axes of different lengths that are all inclined relative to each other.
- Monoclinic: This crystal system has three axes of different lengths, with one axis perpendicular to the other two.
- Rhombohedral: This crystal system has three axes of equal length, all intersecting at angles that are not right angles.
Explanation:
Isotropical is not a valid crystal system because it does not describe a specific arrangement of axes and angles that characterize a crystal structure. It is important to understand the different types of crystal systems in order to study the properties and behavior of crystals in materials science and mineralogy.
The IUPAC name of the compound having the formula (CH₃)₃C-CH =CH₂ is
- a)3,3-Dimethyl-1-butene
- b)3,3,3-Trimethyl-10propene
- c)1,1,1-Trimethyl-1-propene
- d)1,1-Dimethyl-3-butene
Correct answer is option 'A'. Can you explain this answer?
The IUPAC name of the compound having the formula (CH₃)₃C-CH =CH₂ is
a)
3,3-Dimethyl-1-butene
b)
3,3,3-Trimethyl-10propene
c)
1,1,1-Trimethyl-1-propene
d)
1,1-Dimethyl-3-butene
|
|
Neha Kulkarni answered |
Explanation:
The given compound has the formula (CH)C-CH =CH.
Step 1: Identify the longest continuous carbon chain
The longest continuous carbon chain in the given compound is a four-carbon chain, which is a butene.
Step 2: Number the carbon chain
Number the carbon chain in such a way that the double bond gets the lowest possible number. In this case, the double bond is between the second and third carbon atoms.
Step 3: Identify the substituents and their positions
There are two methyl groups attached to the second carbon atom.
Step 4: Write the IUPAC name
The IUPAC name of the given compound is 3,3-Dimethyl-1-butene.
Therefore, option (A) is the correct answer.
The given compound has the formula (CH)C-CH =CH.
Step 1: Identify the longest continuous carbon chain
The longest continuous carbon chain in the given compound is a four-carbon chain, which is a butene.
Step 2: Number the carbon chain
Number the carbon chain in such a way that the double bond gets the lowest possible number. In this case, the double bond is between the second and third carbon atoms.
Step 3: Identify the substituents and their positions
There are two methyl groups attached to the second carbon atom.
Step 4: Write the IUPAC name
The IUPAC name of the given compound is 3,3-Dimethyl-1-butene.
Therefore, option (A) is the correct answer.
The ratio of the molar amounts of H₂S needed to precipitate the metal ions from 20 mL each of 1M Cd(NO₃)₂ and 0.5 M CuSO₄ is- a)1 : 1
- b)2 : 1
- c)1 : 2
- d)Indefinite
Correct answer is option 'B'. Can you explain this answer?
The ratio of the molar amounts of H₂S needed to precipitate the metal ions from 20 mL each of 1M Cd(NO₃)₂ and 0.5 M CuSO₄ is
a)
1 : 1
b)
2 : 1
c)
1 : 2
d)
Indefinite
|
|
Sravya Yadav answered |
As Cd2+ and Cu2+, both are dipositive ions but Cu2+ has one half of the concentration compared to that of the Cd2+. Thus, the molar amount of the H2S used to precipitate the metals ions Cd2+ and Cu2+ is 2 : 1.
The equivalent conductivity of 0.1 M weak acid is 100 times less than that at infinite dilution. The degree of dissociation is- a)100
- b)10
- c)0.01
- d)0.001
Correct answer is option 'C'. Can you explain this answer?
The equivalent conductivity of 0.1 M weak acid is 100 times less than that at infinite dilution. The degree of dissociation is
a)
100
b)
10
c)
0.01
d)
0.001

|
Learners Habitat answered |
To find the degree of dissociation of the weak acid:
- The equivalent conductivity of the weak acid at 0.1 M is stated to be 100 times less than that at infinite dilution.
- At infinite dilution, the conductivity represents complete dissociation of the acid.
- The degree of dissociation (α) can be calculated using the following relationship:
- Degree of dissociation is defined as the fraction of the acid that dissociates in solution.
- If the conductivity at 0.1 M is 1% of the infinite dilution value, the degree of dissociation can be derived as:
- α = (Conductivity at 0.1 M) / (Conductivity at infinite dilution)
- Given that 0.1 M weak acid conductivity is 1% (1/100) of the infinite dilution value, α = 0.01.
The degree of dissociation is thus 0.01.
Ring test for nitrate is performed by adding freshly prepared FeSO₄ solution and conc. H₂SO₄. The brown ring in this test is due to- a)FeSO₄NO₂
- b)FeSO₄NO
- c)FeSO₄
- d)Fe₂(SO₄)₃
Correct answer is option 'B'. Can you explain this answer?
Ring test for nitrate is performed by adding freshly prepared FeSO₄ solution and conc. H₂SO₄. The brown ring in this test is due to
a)
FeSO₄NO₂
b)
FeSO₄NO
c)
FeSO₄
d)
Fe₂(SO₄)₃
|
|
Ashwini Sengupta answered |
Nitrate Test Overview
The ring test for nitrate is a qualitative analysis technique used to detect the presence of nitrate ions (NO3-) in a solution. It involves the use of iron(II) sulfate (FeSO4) and concentrated sulfuric acid (H2SO4).
Procedure
- Add FeSO4: Freshly prepared FeSO4 solution is added to the test tube containing the sample suspected of containing nitrate.
- Add H2SO4: Concentrated H2SO4 is carefully added down the side of the test tube to avoid mixing.
Formation of the Brown Ring
When the two reagents interact, a reaction occurs that leads to the formation of a distinct brown ring at the interface of the two layers. This is crucial for identifying the presence of nitrates.
Explanation of the Brown Ring
- Chemical Reaction: Nitrate ions react with FeSO4 in the presence of concentrated sulfuric acid to form a complex compound.
- Formation of [Fe(SO4)(NO)] Complex: The correct product formed that gives the brown ring is the iron(II) nitrosyl complex, represented as [Fe(SO4)(NO)].
- Brown Color: This complex is responsible for the brown color observed in the ring test, confirming the presence of nitrate ions.
Conclusion
The brown ring is specifically due to option 'b' [Fe(SO4)(NO)], which indicates a positive result for nitrate ions in the solution. This test is a classic method used in qualitative analysis in chemistry, particularly in the context of examinations like JEE.
The ring test for nitrate is a qualitative analysis technique used to detect the presence of nitrate ions (NO3-) in a solution. It involves the use of iron(II) sulfate (FeSO4) and concentrated sulfuric acid (H2SO4).
Procedure
- Add FeSO4: Freshly prepared FeSO4 solution is added to the test tube containing the sample suspected of containing nitrate.
- Add H2SO4: Concentrated H2SO4 is carefully added down the side of the test tube to avoid mixing.
Formation of the Brown Ring
When the two reagents interact, a reaction occurs that leads to the formation of a distinct brown ring at the interface of the two layers. This is crucial for identifying the presence of nitrates.
Explanation of the Brown Ring
- Chemical Reaction: Nitrate ions react with FeSO4 in the presence of concentrated sulfuric acid to form a complex compound.
- Formation of [Fe(SO4)(NO)] Complex: The correct product formed that gives the brown ring is the iron(II) nitrosyl complex, represented as [Fe(SO4)(NO)].
- Brown Color: This complex is responsible for the brown color observed in the ring test, confirming the presence of nitrate ions.
Conclusion
The brown ring is specifically due to option 'b' [Fe(SO4)(NO)], which indicates a positive result for nitrate ions in the solution. This test is a classic method used in qualitative analysis in chemistry, particularly in the context of examinations like JEE.
The volume of 2.8 g of CO at 270C and 0.821 atm. pressure is (R=0.0821 lit. atm mol-1K-1)
- a)1.5 litre
- b)3 litre
- c)30 litre
- d)0.3 litre
Correct answer is option 'B'. Can you explain this answer?
The volume of 2.8 g of CO at 270C and 0.821 atm. pressure is (R=0.0821 lit. atm mol-1K-1)
a)
1.5 litre
b)
3 litre
c)
30 litre
d)
0.3 litre
|
|
Akshay Mukherjee answered |
Given:
Mass of CO = 2.8 g
Temperature = 27°C = 270 K
Pressure = 0.821 atm
Gas constant, R = 0.0821 L atm mol^(-1) K^(-1)
We need to find the volume of CO.
To solve this problem, we can use the ideal gas equation:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = gas constant
T = temperature
Step 1: Calculate the number of moles of CO
To calculate the number of moles, we can use the formula:
n = mass / molar mass
The molar mass of CO = 12.01 g/mol + 16.00 g/mol = 28.01 g/mol
Substituting the values, we get:
n = 2.8 g / 28.01 g/mol
≈ 0.09996 mol
≈ 0.1 mol (rounded to one decimal place)
Step 2: Convert the temperature from Celsius to Kelvin
To convert the temperature from Celsius to Kelvin, we use the formula:
T(K) = T(°C) + 273.15
Substituting the values, we get:
T(K) = 27°C + 273.15
= 300.15 K
Step 3: Substitute the values into the ideal gas equation
Using the ideal gas equation, PV = nRT, we can solve for V:
V = (nRT) / P
Substituting the values, we get:
V = (0.1 mol * 0.0821 L atm mol^(-1) K^(-1) * 300.15 K) / 0.821 atm
≈ 3.002 L
≈ 3 L (rounded to one decimal place)
Therefore, the volume of 2.8 g of CO at 27°C and 0.821 atm pressure is approximately 3 liters, which corresponds to option B.
Mass of CO = 2.8 g
Temperature = 27°C = 270 K
Pressure = 0.821 atm
Gas constant, R = 0.0821 L atm mol^(-1) K^(-1)
We need to find the volume of CO.
To solve this problem, we can use the ideal gas equation:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = gas constant
T = temperature
Step 1: Calculate the number of moles of CO
To calculate the number of moles, we can use the formula:
n = mass / molar mass
The molar mass of CO = 12.01 g/mol + 16.00 g/mol = 28.01 g/mol
Substituting the values, we get:
n = 2.8 g / 28.01 g/mol
≈ 0.09996 mol
≈ 0.1 mol (rounded to one decimal place)
Step 2: Convert the temperature from Celsius to Kelvin
To convert the temperature from Celsius to Kelvin, we use the formula:
T(K) = T(°C) + 273.15
Substituting the values, we get:
T(K) = 27°C + 273.15
= 300.15 K
Step 3: Substitute the values into the ideal gas equation
Using the ideal gas equation, PV = nRT, we can solve for V:
V = (nRT) / P
Substituting the values, we get:
V = (0.1 mol * 0.0821 L atm mol^(-1) K^(-1) * 300.15 K) / 0.821 atm
≈ 3.002 L
≈ 3 L (rounded to one decimal place)
Therefore, the volume of 2.8 g of CO at 27°C and 0.821 atm pressure is approximately 3 liters, which corresponds to option B.
Chapter doubts & questions for Chemistry - VITEEE: Subject Wise and Full Length MOCK Tests 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemistry - VITEEE: Subject Wise and Full Length MOCK Tests in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily











