All Exams >
Mechanical Engineering >
6 Months Preparation for GATE Mechanical >
All Questions
All questions of Introduction to Heat Transfer for Mechanical Engineering Exam
Choose the correct statement about thermaf conductivity- a)thermal conductivity for metals decreases with increase in temperature
- b)thermal conductivity for gases and insulating material decreases with increase in temperature
- c)thermal conductivity is not a function of temperature
- d)thermal conductivity increases with increase in temperature irrespective of material
Correct answer is option 'A'. Can you explain this answer?
Choose the correct statement about thermaf conductivity
a)
thermal conductivity for metals decreases with increase in temperature
b)
thermal conductivity for gases and insulating material decreases with increase in temperature
c)
thermal conductivity is not a function of temperature
d)
thermal conductivity increases with increase in temperature irrespective of material
|
|
Sarita Yadav answered |
Due to increase in lattice vibratioh the thermal conductivity of metals decreases with increase in temperature.
Regarding one dimensional heat transfer, choose the correct statement- a)Steady- f (x), Unsteady- f (x, t)
- b)Steady- f (x, t), Unsteady- f (x)
- c)Steady- f (x, y, t), Unsteady- f (x)
- d)Steady- f (y, z), Unsteady- f (y)
Correct answer is option 'D'. Can you explain this answer?
Regarding one dimensional heat transfer, choose the correct statement
a)
Steady- f (x), Unsteady- f (x, t)
b)
Steady- f (x, t), Unsteady- f (x)
c)
Steady- f (x, y, t), Unsteady- f (x)
d)
Steady- f (y, z), Unsteady- f (y)
|
|
Shounak Mehta answered |
In case of one dimensional heat flow steady state is a function of x coordinate only while unsteady state is a function of x coordinate and time only.
Consider H and O₂ gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that of O₂.
- a)greater
- b)equal
- c)lower
- d)depends upon pressure
Correct answer is option 'A'. Can you explain this answer?
Consider H and O₂ gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that of O₂.
a)
greater
b)
equal
c)
lower
d)
depends upon pressure
|
|
Sarita Yadav answered |
The thermal conductivity of a gaseous medium is dominantly based on diffusion rate, which depends on root mean square velocity.The root mean square velocity for molecules is given by
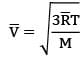
where M is the molecular mass of the gas & T is the temperature of the gas and ̅ is universal gas constant. Thus diffusion rate and therefore thermal conductivity is lower for gases with higher molecular masses because
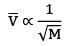
Therefore H₂ gas has more thermal conductivity than O₂.
Consider a light bulb of 100 watts power. When switched on, what is the surface temperature of the tungsten filament in °C. Suppose the surface to be a black surface having value of area equal to 8 x 10-5 m2
- a)2166
- b)1893
- c)800
- d)3786
Correct answer is option 'B'. Can you explain this answer?
Consider a light bulb of 100 watts power. When switched on, what is the surface temperature of the tungsten filament in °C. Suppose the surface to be a black surface having value of area equal to 8 x 10-5 m2
a)
2166
b)
1893
c)
800
d)
3786

|
Telecom Tuners answered |
By Stefan Boltzmann law

T = 2166 K (or) 1893 °C
Convective heat transfer coefficient doesn’t depend on- a)Surface area
- b)Space
- c)Time
- d)Orientation of solid surface
Correct answer is option 'A'. Can you explain this answer?
Convective heat transfer coefficient doesn’t depend on
a)
Surface area
b)
Space
c)
Time
d)
Orientation of solid surface
|
|
Nitin Malik answered |
It is denoted by h and is dependent on space, time, geometry, orientation of solid surface.
With increase in pressure, the thermal conductivity of an ideal gas __________.- a) increases
- b) remains constant
- c) decrease
- d) remains constant
Correct answer is option 'B'. Can you explain this answer?
With increase in pressure, the thermal conductivity of an ideal gas __________.
a)
increases
b)
remains constant
c)
decrease
d)
remains constant
|
|
Sarita Yadav answered |
For an ideal gas, thermal conductivity is given as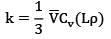
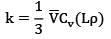
Where ⊽ is the mean travel velocity of molecules Cv, is the specific heat of gas at constant volume, L is the mean free path of molecules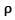 and is the density of the gas.
and is the density of the gas.
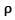 and is the density of the gas.
and is the density of the gas.As pressure increases, density of gas increases but based on experiments it is observed that the mean free path diminishes in the same proportions. Thus the product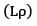 remains constant.
remains constant.
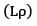 remains constant.
remains constant.Also ⊽ depends upon temperature only hence if temperature is constant, change in pressure does not bring any change in ⊽ Also for an ideal gas Cv is independent of pressure.
Which of the following is an example of forced convection?- a)Chilling effect of cold wind on a warm body
- b)Flow of water in condenser tubes
- c)Cooling of billets in the atmosphere
- d)Heat exchange on cold and warm pipes
Correct answer is option 'B'. Can you explain this answer?
Which of the following is an example of forced convection?
a)
Chilling effect of cold wind on a warm body
b)
Flow of water in condenser tubes
c)
Cooling of billets in the atmosphere
d)
Heat exchange on cold and warm pipes
|
|
Deepak Mehta answered |
In forced convection the flow of fluid is caused by a pump, fan or by atmospheric winds.
For the composite slab shown in figure, find out when k represents thermal conductivity and L represents thickness.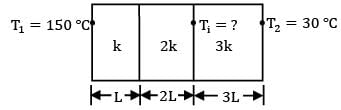
- a) 110
- b) 70
- c) 60
- d) 90
Correct answer is option 'B'. Can you explain this answer?
For the composite slab shown in figure, find out when k represents thermal conductivity and L represents thickness.

a)
110
b)
70
c)
60
d)
90
|
|
Sarita Yadav answered |
The thermal circuit of the system can be represent as follows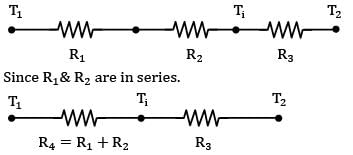
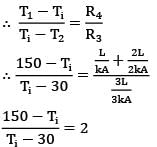

By idea of circuits ratio of resistances is equal to ratio of temperature drops across them
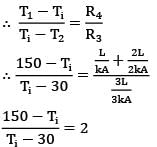
150 - Ti = 2Ti - 60
210 = 3Ti
Ti = 70°C
The literature of heat transfer generally recognizes distinct modes of heat transfer. How many modes are there?- a)One
- b)Two
- c)Three
- d)Four
Correct answer is option 'C'. Can you explain this answer?
The literature of heat transfer generally recognizes distinct modes of heat transfer. How many modes are there?
a)
One
b)
Two
c)
Three
d)
Four
|
|
Malleswari Durgabhavani answered |
Three types are there
1. convection
a) natural convection
b) forced convection
2. conduction
3. radiation
1. convection
a) natural convection
b) forced convection
2. conduction
3. radiation
How many types of convection process are there?- a)One
- b)Three
- c)Four
- d)Two
Correct answer is option 'B'. Can you explain this answer?
How many types of convection process are there?
a)
One
b)
Three
c)
Four
d)
Two
|
|
Shruti Choudhary answered |
Forced, natural and mixed convection.
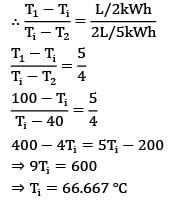
Consider a composite slab as shown in the figure.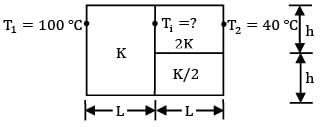 If the left most and right most surfaces are maintained at temperatures of 100°C & 40°C respectively, find out the interface temperature in
If the left most and right most surfaces are maintained at temperatures of 100°C & 40°C respectively, find out the interface temperature in 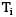 in °CCorrect answer is 'Range: 66 to 67'. Can you explain this answer?
in °CCorrect answer is 'Range: 66 to 67'. Can you explain this answer?
Consider a composite slab as shown in the figure.

If the left most and right most surfaces are maintained at temperatures of 100°C & 40°C respectively, find out the interface temperature in 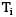 in °C
in °C
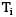 in °C
in °C|
|
Sarita Yadav answered |
The slab can be represented by the following thermal circuit.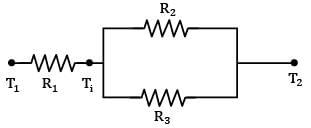
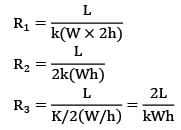
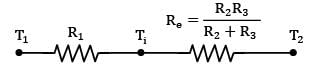
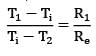
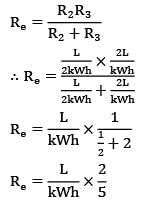

Considering the width of slab to plane of paper to be equal to W,
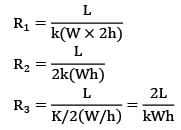
R₂ and R₃ are arranged in parallel and can be shown by single equivalent resistance Rₑ

By idea of series circuit, that ratio of temperature drops across resistances is equal to ratio of respective thermal resistances.
I.e.,
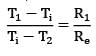


Find out the heat flux (in W/m²) between two fluids separated by a planar wall as shown in the figure.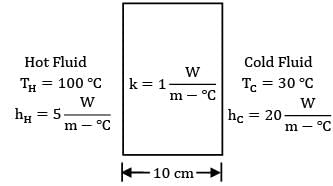
- a)200
- b)200
Correct answer is between '200,200'. Can you explain this answer?
Find out the heat flux (in W/m²) between two fluids separated by a planar wall as shown in the figure.

a)
200
b)
200

|
Telecom Tuners answered |
Consider the thermal circuit for the system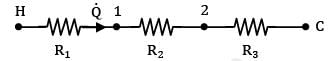
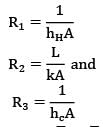
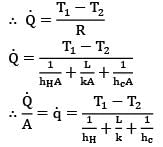
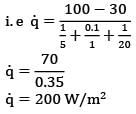

Where nodes H, 1, 2 & C represent hot fluid, left surface of wall, right surface of wall and cold fluid respectively. Therefore,
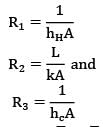
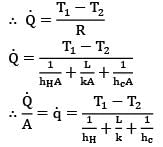
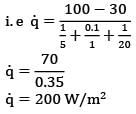
Metals are good conductors of heat because of
- a)all of the below
- b)their atoms are relatively far apart
- c)their atoms collide frequently
- d)free electrons are present
Correct answer is option 'D'. Can you explain this answer?
Metals are good conductors of heat because of
a)
all of the below
b)
their atoms are relatively far apart
c)
their atoms collide frequently
d)
free electrons are present
|
|
Sarita Yadav answered |
- The thermal conductivity of the liquids is more than the gasses and the metals have the highest.
- Metals have valence electrons that are free to move around the surface. When heated, these free electrons collide with each other and the majority of the Kinetic energy is transformed into heat energy, making metals a good conductor of heat.
- In the gaseous state, the molecules of a substance are spaced relatively far away, and their motion is random. This means that energy transfer by the molecular impact is much slower than in the case of a liquid, in which the motion is still random but in liquids, the molecules are more closely packed. The same is true concerning the difference between the thermal conductivity of the liquid and solid phases.
The concept of overall heat transfer is used in the heat transfer in the case of- a)conduction
- b)convection
- c)radiation
- d)combined mode of conduction and convection
Correct answer is option 'D'. Can you explain this answer?
The concept of overall heat transfer is used in the heat transfer in the case of
a)
conduction
b)
convection
c)
radiation
d)
combined mode of conduction and convection

|
Anuj Chakraborty answered |
Note that the concept of overall heat transfer is useful in combined mode of heat transfer like conduction and convection
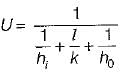
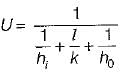
Heat transfer in a long, hollow cylinder which is maintained at uniform but different temperatures on its inner and outer surfaces may be assumed to be taking place in which direction?- a)Axial only
- b)Radial only
- c)Unpredictable
- d)No heat transfer takes place
Correct answer is option 'B'. Can you explain this answer?
Heat transfer in a long, hollow cylinder which is maintained at uniform but different temperatures on its inner and outer surfaces may be assumed to be taking place in which direction?
a)
Axial only
b)
Radial only
c)
Unpredictable
d)
No heat transfer takes place
|
|
Sarita Yadav answered |
Ambient temperature is uniform on the periphery of cylinder and temperature is uniform. So it takes place in the radial direction only.
Heat transfer takes place in liquids and gases is majorly due to
- a)Radiation
- b)Conduction
- c)Convection
- d)Conduction as well as convection
Correct answer is option 'C'. Can you explain this answer?
Heat transfer takes place in liquids and gases is majorly due to
a)
Radiation
b)
Conduction
c)
Convection
d)
Conduction as well as convection
|
|
Gitanjali Iyer answered |
Convection is a process by which thermal energy is transferred between solid and fluid flowing through it.
Thermal conductivity of wood depends on- a)moisture
- b)density
- c)temperature
- d)all of these
Correct answer is option 'D'. Can you explain this answer?
Thermal conductivity of wood depends on
a)
moisture
b)
density
c)
temperature
d)
all of these
|
|
Sanskriti Chakraborty answered |
Thermal conductivity of wood is affected by several factors, including moisture content, density, and temperature. Let's discuss each of these factors in detail:
Moisture content:
- Wood is a hygroscopic material, which means it can absorb or release moisture from the environment.
- Moisture content in wood can affect its thermal conductivity because water has a much higher thermal conductivity than wood.
- As the moisture content in wood increases, its thermal conductivity also increases. This is because the water molecules conduct heat better than the wood molecules.
Density:
- The density of wood can also affect its thermal conductivity.
- Generally, the denser the wood, the higher its thermal conductivity. This is because denser wood has more atoms per unit volume, which means there are more pathways for heat transfer to occur.
Temperature:
- The temperature of wood can also affect its thermal conductivity.
- As the temperature of wood increases, its thermal conductivity also increases. This is because the wood molecules vibrate more rapidly at higher temperatures, which means they can transfer heat more efficiently.
Overall, the thermal conductivity of wood is influenced by these three factors: moisture content, density, and temperature. Therefore, option D, "all of these," is the correct answer.
Moisture content:
- Wood is a hygroscopic material, which means it can absorb or release moisture from the environment.
- Moisture content in wood can affect its thermal conductivity because water has a much higher thermal conductivity than wood.
- As the moisture content in wood increases, its thermal conductivity also increases. This is because the water molecules conduct heat better than the wood molecules.
Density:
- The density of wood can also affect its thermal conductivity.
- Generally, the denser the wood, the higher its thermal conductivity. This is because denser wood has more atoms per unit volume, which means there are more pathways for heat transfer to occur.
Temperature:
- The temperature of wood can also affect its thermal conductivity.
- As the temperature of wood increases, its thermal conductivity also increases. This is because the wood molecules vibrate more rapidly at higher temperatures, which means they can transfer heat more efficiently.
Overall, the thermal conductivity of wood is influenced by these three factors: moisture content, density, and temperature. Therefore, option D, "all of these," is the correct answer.
All the three modes of heat transfer are involved in- a)melting of ice
- b)Cooling of a small metal casting in a quenching bath
- c)heat flow through the walls of a refrigerator
- d)automobile engine equipped with a thermosyphon cooling system
Correct answer is option 'D'. Can you explain this answer?
All the three modes of heat transfer are involved in
a)
melting of ice
b)
Cooling of a small metal casting in a quenching bath
c)
heat flow through the walls of a refrigerator
d)
automobile engine equipped with a thermosyphon cooling system
|
|
Nayanika Yadav answered |
Heat Transfer in Different Processes
Melting of Ice:
- Involves only one mode of heat transfer, i.e. latent heat transfer
- Heat is absorbed by the ice to break the bonds between molecules and change the phase from solid to liquid
- No conduction or convection involved
Cooling of a Small Metal Casting in a Quenching Bath:
- Involves two modes of heat transfer, i.e. convection and conduction
- Metal casting is immersed in a quenching bath to cool it quickly
- Convection occurs as the cooler liquid in the bath comes in contact with the hot metal casting and carries away the heat
- Conduction occurs as heat is transferred from the hot metal casting to the cooler liquid in the bath
Heat Flow Through the Walls of a Refrigerator:
- Involves only one mode of heat transfer, i.e. conduction
- Heat flows from the warmer environment outside the refrigerator to the cooler environment inside the refrigerator
- The walls of the refrigerator act as a barrier to the heat flow through conduction
Automobile Engine Equipped with a Thermosyphon Cooling System:
- Involves all three modes of heat transfer, i.e. conduction, convection, and radiation
- Heat is generated in the engine due to combustion and friction
- Conduction occurs as the heat is transferred from the engine to the coolant in the engine block
- Convection occurs as the heated coolant rises and cooler coolant sinks, creating a natural circulation of the fluid
- Radiation occurs as the heat is transferred from the engine to the surrounding environment
- The thermosyphon cooling system helps in the efficient transfer of heat and keeps the engine cool
Therefore, option 'D' is the correct answer as it involves all three modes of heat transfer.
Melting of Ice:
- Involves only one mode of heat transfer, i.e. latent heat transfer
- Heat is absorbed by the ice to break the bonds between molecules and change the phase from solid to liquid
- No conduction or convection involved
Cooling of a Small Metal Casting in a Quenching Bath:
- Involves two modes of heat transfer, i.e. convection and conduction
- Metal casting is immersed in a quenching bath to cool it quickly
- Convection occurs as the cooler liquid in the bath comes in contact with the hot metal casting and carries away the heat
- Conduction occurs as heat is transferred from the hot metal casting to the cooler liquid in the bath
Heat Flow Through the Walls of a Refrigerator:
- Involves only one mode of heat transfer, i.e. conduction
- Heat flows from the warmer environment outside the refrigerator to the cooler environment inside the refrigerator
- The walls of the refrigerator act as a barrier to the heat flow through conduction
Automobile Engine Equipped with a Thermosyphon Cooling System:
- Involves all three modes of heat transfer, i.e. conduction, convection, and radiation
- Heat is generated in the engine due to combustion and friction
- Conduction occurs as the heat is transferred from the engine to the coolant in the engine block
- Convection occurs as the heated coolant rises and cooler coolant sinks, creating a natural circulation of the fluid
- Radiation occurs as the heat is transferred from the engine to the surrounding environment
- The thermosyphon cooling system helps in the efficient transfer of heat and keeps the engine cool
Therefore, option 'D' is the correct answer as it involves all three modes of heat transfer.
Arrangement of silver, air, aluminium and lead in order of increasing thermal conductivity at room temperature yields- a)Air, Aluminium,'Silver, Lead
- b)Air, Aluminium, Lead, Silver
- c)Lead, Air, Aluminium, Silver
- d)Air, Lead, Aluminium, Silver
Correct answer is option 'D'. Can you explain this answer?
Arrangement of silver, air, aluminium and lead in order of increasing thermal conductivity at room temperature yields
a)
Air, Aluminium,'Silver, Lead
b)
Air, Aluminium, Lead, Silver
c)
Lead, Air, Aluminium, Silver
d)
Air, Lead, Aluminium, Silver

|
Anuj Verma answered |
According to their thermal conductivity for Air, k = 0.022 W/mK
for Aluminium, k = 205 W/mK
for Silver, k = 407 W/mK
Hence correct sequence is
Air, Lead, Aluminium, Silve
for Aluminium, k = 205 W/mK
for Silver, k = 407 W/mK
Hence correct sequence is
Air, Lead, Aluminium, Silve
Consider the following statements:
1. The thermal conductivity of a material is it’s ability to conduct heat.
2. The thermal conductivity can be defined as the rate of heat transfer through a unit thickness of the material per unit area.
3. In solids, heat conduction is due to lattice vibrational energy as well as energy transported via free flow of electrons.
4. Convection involves combined effect of conduction and fluid motion.
Which of the above statements are valid?- a)1 and 2
- b)2, 3 and 4
- c)1, 3 and 4
- d)3 and 4
Correct answer is option 'C'. Can you explain this answer?
Consider the following statements:
1. The thermal conductivity of a material is it’s ability to conduct heat.
2. The thermal conductivity can be defined as the rate of heat transfer through a unit thickness of the material per unit area.
3. In solids, heat conduction is due to lattice vibrational energy as well as energy transported via free flow of electrons.
4. Convection involves combined effect of conduction and fluid motion.
Which of the above statements are valid?
1. The thermal conductivity of a material is it’s ability to conduct heat.
2. The thermal conductivity can be defined as the rate of heat transfer through a unit thickness of the material per unit area.
3. In solids, heat conduction is due to lattice vibrational energy as well as energy transported via free flow of electrons.
4. Convection involves combined effect of conduction and fluid motion.
Which of the above statements are valid?
a)
1 and 2
b)
2, 3 and 4
c)
1, 3 and 4
d)
3 and 4
|
|
Anshul Basu answered |
Thermal conductivity can be defined as the rate of heat transfer through a unit thickness of material per unit area per unit temperature difference.
Thermal conductivity of air with rise in temperature- a)increases
- b)remains constant
- c)decreases
- d)may increase or decrease depending on temperature
Correct answer is option 'A'. Can you explain this answer?
Thermal conductivity of air with rise in temperature
a)
increases
b)
remains constant
c)
decreases
d)
may increase or decrease depending on temperature
|
|
Stuti Bajaj answered |
Thermal conductivity of air with rise in temperature
The correct answer is option 'A', which states that the thermal conductivity of air increases with a rise in temperature. This can be explained by the following factors:
1. Increase in molecular motion:
As the temperature of a gas (such as air) increases, the average kinetic energy of its molecules also increases. This leads to an increase in molecular motion, resulting in more frequent collisions between the molecules. These collisions facilitate the transfer of thermal energy from one molecule to another, thereby increasing the thermal conductivity of the gas.
2. Increase in collision frequency:
With the increase in temperature, the frequency of collisions between gas molecules also increases. This is due to the higher molecular velocity resulting from increased kinetic energy. As a result, there is a greater probability of energy transfer through collisions, leading to higher thermal conductivity.
3. Decrease in mean free path:
The mean free path is the average distance a molecule travels between successive collisions. With the rise in temperature, the mean free path of air molecules decreases. This is because the increased molecular motion leads to more frequent collisions, reducing the average distance traveled by the molecules. A shorter mean free path implies that the molecules have less distance to travel before they collide and transfer thermal energy. Consequently, the thermal conductivity of air increases.
4. Impact of molecular structure:
The molecular structure of air remains relatively unchanged with temperature variations. Therefore, the increase in thermal conductivity is primarily influenced by the factors mentioned above, such as increased molecular motion and collision frequency.
In summary, the thermal conductivity of air increases with a rise in temperature due to enhanced molecular motion, higher collision frequency, and reduced mean free path. These factors contribute to a more efficient transfer of thermal energy within the gas.
The correct answer is option 'A', which states that the thermal conductivity of air increases with a rise in temperature. This can be explained by the following factors:
1. Increase in molecular motion:
As the temperature of a gas (such as air) increases, the average kinetic energy of its molecules also increases. This leads to an increase in molecular motion, resulting in more frequent collisions between the molecules. These collisions facilitate the transfer of thermal energy from one molecule to another, thereby increasing the thermal conductivity of the gas.
2. Increase in collision frequency:
With the increase in temperature, the frequency of collisions between gas molecules also increases. This is due to the higher molecular velocity resulting from increased kinetic energy. As a result, there is a greater probability of energy transfer through collisions, leading to higher thermal conductivity.
3. Decrease in mean free path:
The mean free path is the average distance a molecule travels between successive collisions. With the rise in temperature, the mean free path of air molecules decreases. This is because the increased molecular motion leads to more frequent collisions, reducing the average distance traveled by the molecules. A shorter mean free path implies that the molecules have less distance to travel before they collide and transfer thermal energy. Consequently, the thermal conductivity of air increases.
4. Impact of molecular structure:
The molecular structure of air remains relatively unchanged with temperature variations. Therefore, the increase in thermal conductivity is primarily influenced by the factors mentioned above, such as increased molecular motion and collision frequency.
In summary, the thermal conductivity of air increases with a rise in temperature due to enhanced molecular motion, higher collision frequency, and reduced mean free path. These factors contribute to a more efficient transfer of thermal energy within the gas.
Which of the following is an example of steady state heat transfer?- a)Boilers and turbines
- b)Cooling of I.C engine
- c)Chilling effect of cold wind on a warm body
- d)Electric bulb cools down by the surrounding atmosphere
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an example of steady state heat transfer?
a)
Boilers and turbines
b)
Cooling of I.C engine
c)
Chilling effect of cold wind on a warm body
d)
Electric bulb cools down by the surrounding atmosphere
|
|
Kiran Choudhary answered |
System is a perfect black body.
Most unsteady heat flow occurs- a)Through the walls of the refrigerator
- b)During annealing of castings
- c)Through the walls of the furnace
- d)Through lagged pipe carrying steam
Correct answer is option 'B'. Can you explain this answer?
Most unsteady heat flow occurs
a)
Through the walls of the refrigerator
b)
During annealing of castings
c)
Through the walls of the furnace
d)
Through lagged pipe carrying steam
|
|
Nandini Mishra answered |
Under steady state condition, with time there is a change in temperature i.e. temperature field is a function of space and time.
Heat transfer in a long, hollow cylinder which is maintained at uniform but different temperatures on its inner and outer surfaces may be assumed to be taking place in which direction?- a)Axial only
- b)Unpredictable
- c)Radial only
- d)No heat transfer takes place
Correct answer is option 'C'. Can you explain this answer?
Heat transfer in a long, hollow cylinder which is maintained at uniform but different temperatures on its inner and outer surfaces may be assumed to be taking place in which direction?
a)
Axial only
b)
Unpredictable
c)
Radial only
d)
No heat transfer takes place
|
|
Nayanika Gupta answered |
Heat transfer in a long, hollow cylinder
Heat transfer in a long, hollow cylinder can occur in multiple directions. However, when the inner and outer surfaces of the cylinder are maintained at different temperatures, the heat transfer is primarily radial in nature.
Explanation:
1. Conduction in a hollow cylinder:
Conduction is the transfer of heat through a solid material. In the case of a hollow cylinder, heat transfer occurs primarily through conduction. The temperature difference between the inner and outer surfaces of the cylinder creates a temperature gradient, leading to heat flow from the higher temperature side to the lower temperature side.
2. Radial heat transfer:
When the inner and outer surfaces of the cylinder are maintained at different temperatures, heat transfer primarily occurs in the radial direction. This is because the temperature difference exists across the radial thickness of the cylinder. The heat flows from the region of higher temperature towards the region of lower temperature, resulting in radial heat transfer.
3. Axial heat transfer:
In a long, hollow cylinder, heat transfer in the axial direction is negligible compared to the radial direction. Axial heat transfer occurs due to convection or radiation, but it is typically much smaller in magnitude compared to the radial heat transfer. Therefore, in this scenario, the axial heat transfer can be considered negligible.
4. Unpredictable heat transfer:
The option of unpredictable heat transfer is not applicable in this case. Heat transfer in a long, hollow cylinder with uniform but different temperatures on its inner and outer surfaces can be reasonably predicted to occur primarily in the radial direction.
5. No heat transfer:
The option of no heat transfer is incorrect. Heat transfer occurs whenever there is a temperature difference. In the case of a long, hollow cylinder with different temperatures on its inner and outer surfaces, there will be heat transfer from the higher temperature side to the lower temperature side.
Therefore, the correct answer is option 'C' - Radial only. Heat transfer primarily occurs in the radial direction in a long, hollow cylinder when the inner and outer surfaces are maintained at uniform but different temperatures.
Heat transfer in a long, hollow cylinder can occur in multiple directions. However, when the inner and outer surfaces of the cylinder are maintained at different temperatures, the heat transfer is primarily radial in nature.
Explanation:
1. Conduction in a hollow cylinder:
Conduction is the transfer of heat through a solid material. In the case of a hollow cylinder, heat transfer occurs primarily through conduction. The temperature difference between the inner and outer surfaces of the cylinder creates a temperature gradient, leading to heat flow from the higher temperature side to the lower temperature side.
2. Radial heat transfer:
When the inner and outer surfaces of the cylinder are maintained at different temperatures, heat transfer primarily occurs in the radial direction. This is because the temperature difference exists across the radial thickness of the cylinder. The heat flows from the region of higher temperature towards the region of lower temperature, resulting in radial heat transfer.
3. Axial heat transfer:
In a long, hollow cylinder, heat transfer in the axial direction is negligible compared to the radial direction. Axial heat transfer occurs due to convection or radiation, but it is typically much smaller in magnitude compared to the radial heat transfer. Therefore, in this scenario, the axial heat transfer can be considered negligible.
4. Unpredictable heat transfer:
The option of unpredictable heat transfer is not applicable in this case. Heat transfer in a long, hollow cylinder with uniform but different temperatures on its inner and outer surfaces can be reasonably predicted to occur primarily in the radial direction.
5. No heat transfer:
The option of no heat transfer is incorrect. Heat transfer occurs whenever there is a temperature difference. In the case of a long, hollow cylinder with different temperatures on its inner and outer surfaces, there will be heat transfer from the higher temperature side to the lower temperature side.
Therefore, the correct answer is option 'C' - Radial only. Heat transfer primarily occurs in the radial direction in a long, hollow cylinder when the inner and outer surfaces are maintained at uniform but different temperatures.
Consider system A at uniform temperature t and system B at another uniform temperature T (t > T). Let the two systems be brought into contact and be thermally insulated from their surroundings but not from each other. Energy will flow from system A to system B because of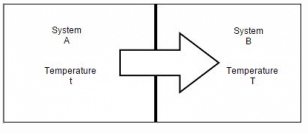
- a)Temperature difference
- b)Energy difference
- c)Mass difference
- d)Volumetric difference
Correct answer is option 'A'. Can you explain this answer?
Consider system A at uniform temperature t and system B at another uniform temperature T (t > T). Let the two systems be brought into contact and be thermally insulated from their surroundings but not from each other. Energy will flow from system A to system B because of
a)
Temperature difference
b)
Energy difference
c)
Mass difference
d)
Volumetric difference
|
|
Rajveer Dasgupta answered |
Greater the temperature imbalance the higher would be the rate of energy transfer.
Which one of the following is correct, in context of thermal diffusivity of liquid and gas- a)αgas > αliquid
- b)αgas < αliquid
- c)αgas = αliquid
- d)Depend on other factors
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is correct, in context of thermal diffusivity of liquid and gas
a)
αgas > αliquid
b)
αgas < αliquid
c)
αgas = αliquid
d)
Depend on other factors

|
Akshat Datta answered |
Since thermal diffusivity, α = k/ρc
(ρC)liquid > (ρC)gas
αgas > αliquid
(ρC)liquid > (ρC)gas
αgas > αliquid
The appropriate rate equation for convective heat transfer between a surface and adjacent fluid is prescribed by- a)Newton’s first law
- b)Wein’s displacement law
- c)Kirchhoff’s law
- d)Newton’s law of cooling
Correct answer is option 'D'. Can you explain this answer?
The appropriate rate equation for convective heat transfer between a surface and adjacent fluid is prescribed by
a)
Newton’s first law
b)
Wein’s displacement law
c)
Kirchhoff’s law
d)
Newton’s law of cooling
|
|
Milan Sen answered |
The rate equation used to describe the mechanism of convection is called Newton’s law of cooling when the solid surface is cooled by fluid.
For a given heat flow rate and for the same thickness, the temperature drop across the material will be maximum for- a) Copper
- b) Steel
- c) Glass-wool
- d) Refractory brick
Correct answer is option 'C'. Can you explain this answer?
For a given heat flow rate and for the same thickness, the temperature drop across the material will be maximum for
a)
Copper
b)
Steel
c)
Glass-wool
d)
Refractory brick
|
|
Aman Kapoor answered |
Explanation:
The temperature drop across a material is determined by its thermal conductivity, which is a measure of how well it conducts heat. The higher the thermal conductivity, the lower the temperature drop for a given heat flow rate and thickness.
Thermal Conductivity:
- Copper has a high thermal conductivity, which means it can conduct heat efficiently. It is commonly used in heat exchangers and electrical wiring.
- Steel also has a relatively high thermal conductivity, although it is lower than copper. It is used in various applications where heat transfer is required.
- Glass-wool is an insulating material and has a low thermal conductivity. It is commonly used for thermal insulation in buildings and industrial applications.
- Refractory brick is also an insulating material and has a low thermal conductivity. It is specifically designed to withstand high temperatures and is used in applications such as furnaces and kilns.
Effect of Thickness:
The thickness of the material does not affect the maximum temperature drop. The temperature drop is determined by the thermal conductivity of the material, which remains constant regardless of thickness.
Comparison:
- Copper and steel have higher thermal conductivities compared to glass-wool and refractory brick.
- Glass-wool and refractory brick have lower thermal conductivities compared to copper and steel.
Conclusion:
Since glass-wool and refractory brick have lower thermal conductivities, they will have a higher temperature drop for a given heat flow rate and thickness. Therefore, option C (Glass-wool) is the correct answer.
The temperature drop across a material is determined by its thermal conductivity, which is a measure of how well it conducts heat. The higher the thermal conductivity, the lower the temperature drop for a given heat flow rate and thickness.
Thermal Conductivity:
- Copper has a high thermal conductivity, which means it can conduct heat efficiently. It is commonly used in heat exchangers and electrical wiring.
- Steel also has a relatively high thermal conductivity, although it is lower than copper. It is used in various applications where heat transfer is required.
- Glass-wool is an insulating material and has a low thermal conductivity. It is commonly used for thermal insulation in buildings and industrial applications.
- Refractory brick is also an insulating material and has a low thermal conductivity. It is specifically designed to withstand high temperatures and is used in applications such as furnaces and kilns.
Effect of Thickness:
The thickness of the material does not affect the maximum temperature drop. The temperature drop is determined by the thermal conductivity of the material, which remains constant regardless of thickness.
Comparison:
- Copper and steel have higher thermal conductivities compared to glass-wool and refractory brick.
- Glass-wool and refractory brick have lower thermal conductivities compared to copper and steel.
Conclusion:
Since glass-wool and refractory brick have lower thermal conductivities, they will have a higher temperature drop for a given heat flow rate and thickness. Therefore, option C (Glass-wool) is the correct answer.
Consider the following materials:
1. Carbon
2. Mica
3. Bakelite
4. Fibreglass
Q. Which of these materials are good conductors- of heat, but bad conductors of electricity- a)1 only
- b)2 only
- c)2 and 3
- d)3 and 4
Correct answer is option 'A'. Can you explain this answer?
Consider the following materials:
1. Carbon
2. Mica
3. Bakelite
4. Fibreglass
Q. Which of these materials are good conductors- of heat, but bad conductors of electricity
1. Carbon
2. Mica
3. Bakelite
4. Fibreglass
Q. Which of these materials are good conductors- of heat, but bad conductors of electricity
a)
1 only
b)
2 only
c)
2 and 3
d)
3 and 4

|
Aditya Jain answered |
Mica, Bakelite and fibre glass are insulator material and carbon in the form of diamond is the good conductor of heat but bad conductor of electricity
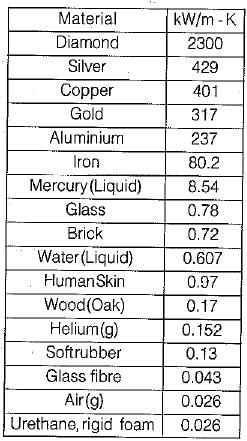
Thermal conductivity is maximum for which substance- a)Silver
- b)Ice
- c)Aluminum
- d)Diamond
Correct answer is option 'D'. Can you explain this answer?
Thermal conductivity is maximum for which substance
a)
Silver
b)
Ice
c)
Aluminum
d)
Diamond
|
|
Aaditya Verma answered |
Thermal conductivity of diamond is 2300 W/m K.
Which of the following events represents thermal radiation?- a) Microwave heating
- b) Nuclear reaction in a sun
- c) Light bulb
- d) None of the above
Correct answer is option 'C'. Can you explain this answer?
Which of the following events represents thermal radiation?
a)
Microwave heating
b)
Nuclear reaction in a sun
c)
Light bulb
d)
None of the above
|
|
Sarita Yadav answered |
In microwave ovens, the radiation emitted is a result of excitation of crystals like magnetrons and klystrons and in microwave wavelength band which is not in the range of thermal radiation. Also during nuclear reactions like those inside sun, -rays are produced which is actually not considered as thermal radiation. Once these -rays get absorbed by the matter in the sun then temperature rises and thermal radiation is emitted. Thus nuclear reaction does not directly emit thermal radiation.
But during lighting of bulb energy is emitted mainly in form of light and IR due to temperature of tungsten filament. Thus this event is termed as thermal radiation.
Which one of the following have a highest thermal conductivity?- a)Boiling water
- b)Steam
- c)Solid ice
- d)Rainwa
Correct answer is option 'C'. Can you explain this answer?
Which one of the following have a highest thermal conductivity?
a)
Boiling water
b)
Steam
c)
Solid ice
d)
Rainwa
|
|
Sanskriti Chakraborty answered |
Understanding Thermal Conductivity
Thermal conductivity is a measure of a material's ability to conduct heat. It varies significantly across different states of matter (solid, liquid, gas).
Factors Affecting Thermal Conductivity
- State of Matter: Solids generally have higher thermal conductivity than liquids and gases due to closely packed molecules.
- Molecular Structure: The arrangement and bonding of atoms affect how easily heat can be transferred.
Comparison of Options
- Boiling Water: While water can conduct heat, its thermal conductivity is lower than that of solids. Its value is about 0.6 W/m·K.
- Steam: As a gas, steam has a lower thermal conductivity compared to liquids and solids. The thermal conductivity of steam is around 0.02 W/m·K.
- Solid Ice: Ice, being a solid, has a higher thermal conductivity than water in liquid form. The thermal conductivity of ice is approximately 2.2 W/m·K.
- Rainwater: Similar to boiling water, rainwater has low thermal conductivity, comparable to that of boiling water.
Conclusion
Based on the comparison, solid ice (option C) possesses the highest thermal conductivity among the listed options. This is due to its solid state, which allows for efficient heat transfer through tightly packed molecular structures. In contrast, the other options (boiling water, steam, and rainwater) are either in liquid or gaseous states, resulting in lower thermal conductivity.
Thus, the correct answer is indeed option C: Solid Ice.
Thermal conductivity is a measure of a material's ability to conduct heat. It varies significantly across different states of matter (solid, liquid, gas).
Factors Affecting Thermal Conductivity
- State of Matter: Solids generally have higher thermal conductivity than liquids and gases due to closely packed molecules.
- Molecular Structure: The arrangement and bonding of atoms affect how easily heat can be transferred.
Comparison of Options
- Boiling Water: While water can conduct heat, its thermal conductivity is lower than that of solids. Its value is about 0.6 W/m·K.
- Steam: As a gas, steam has a lower thermal conductivity compared to liquids and solids. The thermal conductivity of steam is around 0.02 W/m·K.
- Solid Ice: Ice, being a solid, has a higher thermal conductivity than water in liquid form. The thermal conductivity of ice is approximately 2.2 W/m·K.
- Rainwater: Similar to boiling water, rainwater has low thermal conductivity, comparable to that of boiling water.
Conclusion
Based on the comparison, solid ice (option C) possesses the highest thermal conductivity among the listed options. This is due to its solid state, which allows for efficient heat transfer through tightly packed molecular structures. In contrast, the other options (boiling water, steam, and rainwater) are either in liquid or gaseous states, resulting in lower thermal conductivity.
Thus, the correct answer is indeed option C: Solid Ice.
A radiator in a domestic heating system operates at a surface temperature of 60 degree Celsius. Calculate the heat flux at the surface of the radiator if it behaves as a black body- a)697.2 W/m2
- b)786.9 W/m2
- c)324.7 W/m2
- d)592.1 W/m2
Correct answer is option 'A'. Can you explain this answer?
A radiator in a domestic heating system operates at a surface temperature of 60 degree Celsius. Calculate the heat flux at the surface of the radiator if it behaves as a black body
a)
697.2 W/m2
b)
786.9 W/m2
c)
324.7 W/m2
d)
592.1 W/m2
|
|
Rohit Banerjee answered |
Here's the solution to your question:
As, q = Q/A = 5.67 * 10-8 (273+60)4 = 697.2.
Hence, the correct answer is Option A
You can go through these courses of Chemical Engineering for a complete understanding.
If temperature of water increases then thermal conductivity of water
- a)increases continuously
- b)remains constant
- c)decreases continuously
- d)initially increases, achieves a maxima and then decreases
Correct answer is option 'D'. Can you explain this answer?
If temperature of water increases then thermal conductivity of water
a)
increases continuously
b)
remains constant
c)
decreases continuously
d)
initially increases, achieves a maxima and then decreases
|
|
Sarita Yadav answered |
Though for liquids, thermal conductivity decreases with rise in temperature but water is an exceptional case due to bonding between oxygen atom of one molecule and hydrogen atom of other. As a result with rise in temperature, thermal conductivity of water increases from 0°C TO 4,4°C achieves a maxima and then decreases.
The ratio of thermal conductivity to electrical conductivity is equal to- a)Prandtl number
- b)Schmidt number
- c)Lorentz number
- d)Lewis number
Correct answer is option 'C'. Can you explain this answer?
The ratio of thermal conductivity to electrical conductivity is equal to
a)
Prandtl number
b)
Schmidt number
c)
Lorentz number
d)
Lewis number

|
Aniket Pillai answered |
The ratio of thermal conductivity to electrical conductivity is always constant for all the conductors at the given temperature. This is know as Wideman and Frange law.
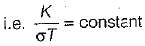
The constant is known as Lorentz number (Lo) whose value is equal to 2.045 x 10-8 W/μK2.
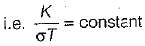
The constant is known as Lorentz number (Lo) whose value is equal to 2.045 x 10-8 W/μK2.
The rate equation used to describe the mechanism of convection is called Newton’s law of cooling. So rate of heat flow by convection doesn’t depend on- a)Convective heat transfer coefficient
- b)Surface area through which heat flows
- c)Time
- d)Temperature potential difference
Correct answer is option 'C'. Can you explain this answer?
The rate equation used to describe the mechanism of convection is called Newton’s law of cooling. So rate of heat flow by convection doesn’t depend on
a)
Convective heat transfer coefficient
b)
Surface area through which heat flows
c)
Time
d)
Temperature potential difference
|
|
Rajveer Dasgupta answered |
It is directly proportional to all of above except time.
An oil cooler in a high performance engine has an outside surface area 0.12 m2 and a surface temperature of 65 degree Celsius. At any intermediate time air moves over the surface of the cooler at a temperature of 30 degree Celsius and gives rise to a surface coefficient equal to 45.4 W/ m 2 K. Find out the heat transfer rate?- a)238.43 W
- b)190.68 W
- c)543.67 W
- d)675.98 W
Correct answer is option 'B'. Can you explain this answer?
An oil cooler in a high performance engine has an outside surface area 0.12 m2 and a surface temperature of 65 degree Celsius. At any intermediate time air moves over the surface of the cooler at a temperature of 30 degree Celsius and gives rise to a surface coefficient equal to 45.4 W/ m 2 K. Find out the heat transfer rate?
a)
238.43 W
b)
190.68 W
c)
543.67 W
d)
675.98 W
|
|
Aaditya Verma answered |
: Q = (T2 – T1) A h = 0.12 (65-30) 45.4 = 190.68 W.
Cork is a good insulator because- a) It is flexible
- b) It can be powered
- c) Low density
- d) It is porous
Correct answer is option 'D'. Can you explain this answer?
Cork is a good insulator because
a)
It is flexible
b)
It can be powered
c)
Low density
d)
It is porous
|
|
Sarita Yadav answered |
Even though cork is a solid material, but it’s thermal conductivity is extremely low because of air filled in it’s pores. Typical value of thermal conductivity is in the range of 0.035 to 0.043 W/m-k
In which of the following material non-isotropic conductivity is exhibited- a)Lead
- b)Wood
- c)Copper
- d)Brass
Correct answer is option 'B'. Can you explain this answer?
In which of the following material non-isotropic conductivity is exhibited
a)
Lead
b)
Wood
c)
Copper
d)
Brass

|
Rajat Patel answered |
Wood is considered as orthotropic material since property of grain along the wood is different and across the grain is different.
A satellite float in deep space with very high velocity. It will continuously lose heat by- a)convection
- b)conduction and convection
- c)radiation
- d)radiation and convection
Correct answer is option 'C'. Can you explain this answer?
A satellite float in deep space with very high velocity. It will continuously lose heat by
a)
convection
b)
conduction and convection
c)
radiation
d)
radiation and convection
|
|
Kalyan Chakraborty answered |
Explanation:
When an object is in space, it is not in contact with any material substance to transfer heat by conduction or convection. Therefore, the only way for the heat to escape from the object is through radiation. This is because all objects emit electromagnetic radiation in the form of infrared radiation, which carries away the heat energy from the object.
In the case of a satellite in deep space, the satellite is subjected to extreme temperatures due to the lack of atmosphere to insulate it from the sun's radiation. Therefore, the satellite must have a way to dissipate the heat that is generated by its electronic systems to prevent them from overheating and failing.
Radiation is the only way for the satellite to lose heat in space. Therefore, the satellite is equipped with thermal radiators, which are designed to radiate the heat away from the satellite's electronic systems into space. These radiators are made of materials that are good at emitting infrared radiation and are positioned in such a way as to maximize the amount of heat that is radiated away from the satellite.
Conclusion:
Therefore, the correct answer is option 'C' - radiation.
When an object is in space, it is not in contact with any material substance to transfer heat by conduction or convection. Therefore, the only way for the heat to escape from the object is through radiation. This is because all objects emit electromagnetic radiation in the form of infrared radiation, which carries away the heat energy from the object.
In the case of a satellite in deep space, the satellite is subjected to extreme temperatures due to the lack of atmosphere to insulate it from the sun's radiation. Therefore, the satellite must have a way to dissipate the heat that is generated by its electronic systems to prevent them from overheating and failing.
Radiation is the only way for the satellite to lose heat in space. Therefore, the satellite is equipped with thermal radiators, which are designed to radiate the heat away from the satellite's electronic systems into space. These radiators are made of materials that are good at emitting infrared radiation and are positioned in such a way as to maximize the amount of heat that is radiated away from the satellite.
Conclusion:
Therefore, the correct answer is option 'C' - radiation.
In which one of the following materials, is the heat energy propagation minimum due to conduction heat transfer- a)Lead
- b)Copper
- c)Water
- d)Air
Correct answer is option 'D'. Can you explain this answer?
In which one of the following materials, is the heat energy propagation minimum due to conduction heat transfer
a)
Lead
b)
Copper
c)
Water
d)
Air

|
Bhargavi Kulkarni answered |
Understanding Heat Transfer by Conduction
Heat transfer occurs through various mechanisms, one of which is conduction. The efficiency of conduction depends on the material's properties, especially its thermal conductivity.
Thermal Conductivity Overview
- Definition: Thermal conductivity is a measure of a material's ability to conduct heat.
- Materials Comparison: Different materials have varying levels of thermal conductivity, influencing how quickly they can transfer heat.
Materials and Their Thermal Conductivity
- Lead: Has a relatively low thermal conductivity but is still better than air.
- Copper: Known for high thermal conductivity, making it an excellent heat conductor.
- Water: While fluid, it also conducts heat reasonably well, especially compared to gases.
- Air: Has the lowest thermal conductivity among the listed options.
Why Air Propagates Heat Minimum by Conduction
- Thermal Conductivity of Air: Air has a thermal conductivity of about 0.025 W/m·K, which is significantly lower than that of solids and liquids.
- Molecular Structure: In gases, molecules are farther apart, which means they collide less frequently, resulting in slower heat transfer.
- Insulating Properties: Due to its low density and molecular spacing, air acts as an insulator, making it less effective at transferring heat via conduction.
Conclusion
Among the materials listed, air is the least capable of conducting heat due to its low thermal conductivity. This property makes air ideal for applications requiring thermal insulation, confirming that option 'D' is indeed correct.
Heat transfer occurs through various mechanisms, one of which is conduction. The efficiency of conduction depends on the material's properties, especially its thermal conductivity.
Thermal Conductivity Overview
- Definition: Thermal conductivity is a measure of a material's ability to conduct heat.
- Materials Comparison: Different materials have varying levels of thermal conductivity, influencing how quickly they can transfer heat.
Materials and Their Thermal Conductivity
- Lead: Has a relatively low thermal conductivity but is still better than air.
- Copper: Known for high thermal conductivity, making it an excellent heat conductor.
- Water: While fluid, it also conducts heat reasonably well, especially compared to gases.
- Air: Has the lowest thermal conductivity among the listed options.
Why Air Propagates Heat Minimum by Conduction
- Thermal Conductivity of Air: Air has a thermal conductivity of about 0.025 W/m·K, which is significantly lower than that of solids and liquids.
- Molecular Structure: In gases, molecules are farther apart, which means they collide less frequently, resulting in slower heat transfer.
- Insulating Properties: Due to its low density and molecular spacing, air acts as an insulator, making it less effective at transferring heat via conduction.
Conclusion
Among the materials listed, air is the least capable of conducting heat due to its low thermal conductivity. This property makes air ideal for applications requiring thermal insulation, confirming that option 'D' is indeed correct.
Which statement is true regarding steady state condition?- a)There is a variation in temperature in the course of time
- b)Heat exchange is constant
- c)It is a function of space and time coordinates
- d)Internal energy of the system changes
Correct answer is option 'B'. Can you explain this answer?
Which statement is true regarding steady state condition?
a)
There is a variation in temperature in the course of time
b)
Heat exchange is constant
c)
It is a function of space and time coordinates
d)
Internal energy of the system changes
|
|
Gitanjali Iyer answered |
Heat influx is always equal to heat efflux. It is a function of space coordinates only.
Heat is mainly transferred by conduction, convection and radiation in- a)insulated pipes carrying hot water
- b)refrigerator freezer coil
- c)boiler furnace
- d)condensation of steam in a condenser
Correct answer is option 'C'. Can you explain this answer?
Heat is mainly transferred by conduction, convection and radiation in
a)
insulated pipes carrying hot water
b)
refrigerator freezer coil
c)
boiler furnace
d)
condensation of steam in a condenser

|
Meghana Desai answered |
Because for radiation to be comparable the magnitude of temperature difference should be large enough, Convection & conduction is also predominant in boiler furnace.
Consider the astronaut compartments inside a space shuttle orbiting the moon. Inside these compartments air is maintained at STP conditions for comfortable breathing of astronauts. The cooling system of electronic equipment inside these compartments is based on- a) Conduction
- b) Forced convection
- c) Free convection
- d) Thermal radiation
Correct answer is option 'B'. Can you explain this answer?
Consider the astronaut compartments inside a space shuttle orbiting the moon. Inside these compartments air is maintained at STP conditions for comfortable breathing of astronauts. The cooling system of electronic equipment inside these compartments is based on
a)
Conduction
b)
Forced convection
c)
Free convection
d)
Thermal radiation
|
|
Sarita Yadav answered |
Since the equipment is in air and surface temperature is generally not high, conduction and radiation heat loss is insignificant. Also in space there is no gravity. Thus buoyancy and therefore free convection is not at all possible in the compartment air. Hence the only suitable mode for cooling is forced convection.
The propagation of heat by molecular level activity is called- a) Convection
- b) Conduction
- c) Radiation
- d) Ionization
Correct answer is option 'B'. Can you explain this answer?
The propagation of heat by molecular level activity is called
a)
Convection
b)
Conduction
c)
Radiation
d)
Ionization
|
|
Sarita Yadav answered |
Heat conduction through a medium is a result of molecular level activities in the medium which are 1. Lattice vibration 2. Free transfer 3. Diffusion and 4. Intermolecular collision when accompanied by a temperature gradient.
Temperature of steam at around 540°C can be measured by- a)thermometer
- b)radiation pyrometer
- c)thermistor
- d)thermocouple
Correct answer is option 'D'. Can you explain this answer?
Temperature of steam at around 540°C can be measured by
a)
thermometer
b)
radiation pyrometer
c)
thermistor
d)
thermocouple
|
|
Shruti Bose answered |
°F (282°C) and a pressure of 1000 psi (6.9 MPa) is approximately 621°F (327°C).
A sphere, a cube and a disc all are of the same material, quality and volume, are heated to 900 K and left in air. Which of these will have the lowest rate of cooling- a) Cube
- b) Disc
- c) Sphere
- d) All will have the same rate of cooling
Correct answer is option 'C'. Can you explain this answer?
A sphere, a cube and a disc all are of the same material, quality and volume, are heated to 900 K and left in air. Which of these will have the lowest rate of cooling
a)
Cube
b)
Disc
c)
Sphere
d)
All will have the same rate of cooling
|
|
Nilotpal Sharma answered |
Rate of Cooling of Different Shapes
When an object is heated and left in air, it will gradually cool down as it loses heat to the surrounding environment. The rate at which this cooling occurs depends on several factors, including the shape of the object. In this case, we have a sphere, a cube, and a disc, all made of the same material, quality, and volume, and heated to 900 K. We need to determine which of these shapes will have the lowest rate of cooling.
Surface Area and Cooling
The rate of cooling of an object is directly proportional to its surface area. Objects with larger surface areas will cool down faster than those with smaller surface areas. This is because a larger surface area allows for more heat transfer between the object and its surroundings.
Comparison of Shapes
Cube: A cube has six identical square faces, so its surface area is given by 6 * side^2, where side is the length of the cube's side. The cube has a total surface area of 6a^2, where a is the side length.
Disc: A disc has a circular shape, and its surface area is given by π * radius^2, where π is a constant (approximately 3.14) and radius is the distance from the center of the disc to its outer edge.
Sphere: A sphere has a curved surface, and its surface area is given by 4 * π * radius^2.
Comparison of Surface Areas
To compare the surface areas of the cube, disc, and sphere, we need to consider their volumes. Since the objects have the same volume, we can equate their respective formulas:
6a^2 = π * radius^2 = 4 * π * radius^2
Simplifying this equation, we find:
6a^2 = 4 * π * radius^2
Since the objects have the same volume, their side lengths and radii are related as follows:
a^3 = (4/3) * π * radius^3
Conclusion
From the equation above, we can see that the radius of the sphere is greater than the side length of the cube or the radius of the disc. As a result, the sphere has the largest surface area, followed by the disc, and finally the cube.
Since the rate of cooling is directly proportional to the surface area, the sphere will have the highest rate of cooling, followed by the disc, and finally the cube. Therefore, the correct answer is option 'C' - the sphere will have the lowest rate of cooling.
When an object is heated and left in air, it will gradually cool down as it loses heat to the surrounding environment. The rate at which this cooling occurs depends on several factors, including the shape of the object. In this case, we have a sphere, a cube, and a disc, all made of the same material, quality, and volume, and heated to 900 K. We need to determine which of these shapes will have the lowest rate of cooling.
Surface Area and Cooling
The rate of cooling of an object is directly proportional to its surface area. Objects with larger surface areas will cool down faster than those with smaller surface areas. This is because a larger surface area allows for more heat transfer between the object and its surroundings.
Comparison of Shapes
Cube: A cube has six identical square faces, so its surface area is given by 6 * side^2, where side is the length of the cube's side. The cube has a total surface area of 6a^2, where a is the side length.
Disc: A disc has a circular shape, and its surface area is given by π * radius^2, where π is a constant (approximately 3.14) and radius is the distance from the center of the disc to its outer edge.
Sphere: A sphere has a curved surface, and its surface area is given by 4 * π * radius^2.
Comparison of Surface Areas
To compare the surface areas of the cube, disc, and sphere, we need to consider their volumes. Since the objects have the same volume, we can equate their respective formulas:
6a^2 = π * radius^2 = 4 * π * radius^2
Simplifying this equation, we find:
6a^2 = 4 * π * radius^2
Since the objects have the same volume, their side lengths and radii are related as follows:
a^3 = (4/3) * π * radius^3
Conclusion
From the equation above, we can see that the radius of the sphere is greater than the side length of the cube or the radius of the disc. As a result, the sphere has the largest surface area, followed by the disc, and finally the cube.
Since the rate of cooling is directly proportional to the surface area, the sphere will have the highest rate of cooling, followed by the disc, and finally the cube. Therefore, the correct answer is option 'C' - the sphere will have the lowest rate of cooling.
Identify the wrong statement- a)The process of heat transfer is an irreversible process
- b)For heat exchange, a temperature gradient must exist
- c)A material medium is not necessary for heat transmission
- d)Heat flow doesn’t depends on temperature
Correct answer is option 'D'. Can you explain this answer?
Identify the wrong statement
a)
The process of heat transfer is an irreversible process
b)
For heat exchange, a temperature gradient must exist
c)
A material medium is not necessary for heat transmission
d)
Heat flow doesn’t depends on temperature
|
|
Ishani Kaur answered |
Heat flows from higher to lower temperature.
Unit of rate of heat transfer is- a)Joule
- b)Newton
- c)Pascal
- d)Watt
Correct answer is option 'D'. Can you explain this answer?
Unit of rate of heat transfer is
a)
Joule
b)
Newton
c)
Pascal
d)
Watt
|
|
Manoj Sharma answered |
Unit of rate of heat transfer is Watt.
Explanation:
Heat transfer is the process of energy transfer from one body or system to another due to a temperature difference between them. It occurs through three main modes: conduction, convection, and radiation. The rate at which heat is transferred is an important parameter in many engineering applications.
The rate of heat transfer is defined as the amount of heat transferred per unit time. It is commonly expressed in units of watts (W), which is a measure of power. Power is the rate at which work is done or energy is transferred. In the context of heat transfer, power refers to the rate at which heat energy is transferred.
The unit of watt is derived from the basic SI units of length, mass, and time. It is defined as one joule per second (1 W = 1 J/s). A joule is the unit of energy, and when heat energy is transferred at the rate of 1 joule per second, it is equivalent to 1 watt.
In other words, if a system or body is transferring heat energy at a rate of 1 watt, it means that 1 joule of heat energy is being transferred every second. This rate can be higher or lower depending on the specific application. For example, a small electronic device may transfer heat at a rate of a few watts, while a large industrial process may involve heat transfer in the range of hundreds or thousands of watts.
In conclusion, the unit of rate of heat transfer is the watt, which represents the rate at which heat energy is transferred.
Explanation:
Heat transfer is the process of energy transfer from one body or system to another due to a temperature difference between them. It occurs through three main modes: conduction, convection, and radiation. The rate at which heat is transferred is an important parameter in many engineering applications.
The rate of heat transfer is defined as the amount of heat transferred per unit time. It is commonly expressed in units of watts (W), which is a measure of power. Power is the rate at which work is done or energy is transferred. In the context of heat transfer, power refers to the rate at which heat energy is transferred.
The unit of watt is derived from the basic SI units of length, mass, and time. It is defined as one joule per second (1 W = 1 J/s). A joule is the unit of energy, and when heat energy is transferred at the rate of 1 joule per second, it is equivalent to 1 watt.
In other words, if a system or body is transferring heat energy at a rate of 1 watt, it means that 1 joule of heat energy is being transferred every second. This rate can be higher or lower depending on the specific application. For example, a small electronic device may transfer heat at a rate of a few watts, while a large industrial process may involve heat transfer in the range of hundreds or thousands of watts.
In conclusion, the unit of rate of heat transfer is the watt, which represents the rate at which heat energy is transferred.
Chapter doubts & questions for Introduction to Heat Transfer - 6 Months Preparation for GATE Mechanical 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Introduction to Heat Transfer - 6 Months Preparation for GATE Mechanical in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
6 Months Preparation for GATE Mechanical
499 videos|1037 docs|710 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup