All Exams >
MCAT >
MCAT Chemical and Physical Foundations >
All Questions
All questions of Gas Phase (GC, PHY) for MCAT Exam
The amount of work done in moving a charge from one point to another along an equipotential line or surface charge is- a)Zero
- b)Infinity
- c)One
- d)Two
Correct answer is option 'A'. Can you explain this answer?
The amount of work done in moving a charge from one point to another along an equipotential line or surface charge is
a)
Zero
b)
Infinity
c)
One
d)
Two
|
|
Om Desai answered |
Since Potential difference between two points in equipotential surfaces is zero, the work done between two points in equipotential surface is also zero.
Work done in carrying 2C charge in a circular path of radius 2m around a charge of 10C is- a)6.67J
- b)60J
- c)Zero
- d)15J
Correct answer is option 'C'. Can you explain this answer?
Work done in carrying 2C charge in a circular path of radius 2m around a charge of 10C is
a)
6.67J
b)
60J
c)
Zero
d)
15J
|
|
Suresh Iyer answered |
The overall work performed in carrying a 2coulomb charge in a circular orbit of radius 3 m around a charge of 10 coulomb is calculated below.
It is a well-known fact that W=qdv.
Here dV is the change in overall potential. In the circular orbit of r potential at each point is similar.
Most significantly, the value of r is 3.
The value of dv=0 and hence W=q0=0.
It is a well-known fact that W=qdv.
Here dV is the change in overall potential. In the circular orbit of r potential at each point is similar.
Most significantly, the value of r is 3.
The value of dv=0 and hence W=q0=0.
Electric field intensity at point ‘B’ due to a point charge ‘Q’ kept at a point ‘A’ is 12 NC-1 and the electric potential at a point ‘B’ due to same charge is 6 JC-1. The distance between AB is- a)2 m
- b)1.5 m
- c)1 m
- d)0.5 m
Correct answer is option 'D'. Can you explain this answer?
Electric field intensity at point ‘B’ due to a point charge ‘Q’ kept at a point ‘A’ is 12 NC-1 and the electric potential at a point ‘B’ due to same charge is 6 JC-1. The distance between AB is
a)
2 m
b)
1.5 m
c)
1 m
d)
0.5 m
|
|
Nandini Patel answered |
As we know , E . l = V where,
E = electric field intensity = 12 N/C
V = electric potential = 6 J/C
=> distance between A and B ,
l = ( 6 / 12 ) m or ( 1 / 2 ) m = 0.5 m
If 100 J of work has to be done in moving an electric charge of 4C from a place where potential is -5 V to another place, where potential is V volt. The value of V is- a)15 V
- b)20 V
- c)25 V
- d)10 V
Correct answer is option 'B'. Can you explain this answer?
If 100 J of work has to be done in moving an electric charge of 4C from a place where potential is -5 V to another place, where potential is V volt. The value of V is
a)
15 V
b)
20 V
c)
25 V
d)
10 V
|
|
Suresh Iyer answered |
From the definition, the work done to a test charge ‘q0’ from one place to another place in an electric field is given by the formula
W=q0x[vfinal-vinitial ]
100=4x[v-(-5)]
v+5=25
v=20V
W=q0x[vfinal-vinitial ]
100=4x[v-(-5)]
v+5=25
v=20V
Can you explain the answer of this question below:Equal charges are given to two spheres of different radii. The potential will
- A:
be equal on both the spheres
- B:
be more on the smaller sphere
- C:
be more on the bigger sphere
- D:
depend on the material of the sphere
The answer is b.
Equal charges are given to two spheres of different radii. The potential will
be equal on both the spheres
be more on the smaller sphere
be more on the bigger sphere
depend on the material of the sphere

|
Husna answered |
V is inversely proportional to radius
The electrostatic potential energy between two charges q1 and q2 separated by a distance by r is given by- a)
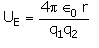
- b)
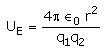
- c)
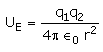
- d)
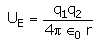
Correct answer is option 'D'. Can you explain this answer?
The electrostatic potential energy between two charges q1 and q2 separated by a distance by r is given by
a)
b)
c)
d)

|
Khushi Mittal answered |
Energy = force × distance U=Kq1q2/r^2 × r U=Kq1q2/r
A hollow metal sphere of radius 5cm is charged so that the potential on its surface is 10V. The potential at a distance of 2cm from the centre of the sphere is- a)4V
- b)zero
- c)10/3V
- d)10V
Correct answer is option 'D'. Can you explain this answer?
A hollow metal sphere of radius 5cm is charged so that the potential on its surface is 10V. The potential at a distance of 2cm from the centre of the sphere is
a)
4V
b)
zero
c)
10/3V
d)
10V

|
Ayush Joshi answered |
In the case of a hollow metal sphere (spherical shell), the electric field inside the shell is zero. This means that the potential inside the shell is constant. Therefore the potential at the centre of the sphere is the same as that on its surface, i.e. 10 V.
Can you explain the answer of this question below:It requires 4 J of work to move a charge of 20 C from point A to point B, separated by a distance of 0.2 cm. The potential difference between A and B in volts
- A:
0.2
- B:
16
- C:
5
- D:
80
The answer is a.
It requires 4 J of work to move a charge of 20 C from point A to point B, separated by a distance of 0.2 cm. The potential difference between A and B in volts
0.2
16
5
80

|
Mayank Singh answered |
As we know tha..v=w/q. .....1 so according to question w=4j and q=20c on putting value of w and q in equation 1 we find that v=0.2v
A hollow metal sphere of radius 10 cm is charged such that the potential on its surface is 80 volt. The potential at the centre of the sphere is- a)8 volt
- b)zero
- c)800 volt
- d)80 volt
Correct answer is option 'D'. Can you explain this answer?
A hollow metal sphere of radius 10 cm is charged such that the potential on its surface is 80 volt. The potential at the centre of the sphere is
a)
8 volt
b)
zero
c)
800 volt
d)
80 volt

|
Sunidhi Pandey answered |
Chage is uniformly distributed in an hollow sphere.
Electric potential is- a)scalar and dimensionless
- b)vector and dimensionless
- c)scalar with dimension
- d)vector with dimension
Correct answer is option 'C'. Can you explain this answer?
Electric potential is
a)
scalar and dimensionless
b)
vector and dimensionless
c)
scalar with dimension
d)
vector with dimension
|
|
Pooja Mehta answered |
He electric potential due to a system of point charges is equal to the sum of the point charges' individual potentials. This fact simplifies calculations significantly, since addition of potential (scalar) fields is much easier than addition of the electric (vector) fields.
The amount of work done in moving a unit positive charge through distance of 10 cm on an equipotential surface is- a)100 joule
- b)10 cm
- c)1/10 cm
- d)Zero
Correct answer is option 'D'. Can you explain this answer?
The amount of work done in moving a unit positive charge through distance of 10 cm on an equipotential surface is
a)
100 joule
b)
10 cm
c)
1/10 cm
d)
Zero

|
Anupam Singh answered |
Zero because at equipotential surface potential difference is zero
Can you explain the answer of this question below:The potential energy of a system containing only one point charge is
- A:
Zero
- B:
Infinity
- C:
Nonzero finite
- D:
None of the above
The answer is a.
The potential energy of a system containing only one point charge is
Zero
Infinity
Nonzero finite
None of the above

|
.mie. answered |
Answer is 0 as there are no other sources of electrostatic potential .... against which an external agent must do work.... in moving the point charge.... from infinity to its final location.... therefore correct opt is A
Consider a solid cube made up of insulating material having a uniform volume charge density. Assuming the electrostatic potential to be zero at infinity, the ratio of the potential at a corner of the cube to that at the centre will be - a)1:1
- b)1:2
- c)1:4
- d)1:8
Correct answer is option 'B'. Can you explain this answer?
Consider a solid cube made up of insulating material having a uniform volume charge density. Assuming the electrostatic potential to be zero at infinity, the ratio of the potential at a corner of the cube to that at the centre will be
a)
1:1
b)
1:2
c)
1:4
d)
1:8
|
|
Sanaya Kumar answered |


By dimensional analysis
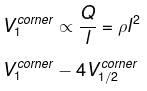
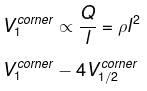
but by superposition
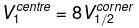
Because of the centre of the larger cube lies at a corner of the eight smaller cubes of which it is made
therefore,
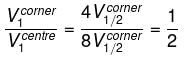
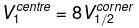
Because of the centre of the larger cube lies at a corner of the eight smaller cubes of which it is made
therefore,
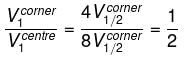
What is the direction of the lines of force at any point on the equipotential surface?- a)Parallel to it
- b)Perpendicular to it.
- c)Inclined at 45 degrees
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
What is the direction of the lines of force at any point on the equipotential surface?
a)
Parallel to it
b)
Perpendicular to it.
c)
Inclined at 45 degrees
d)
None of the above
|
|
Anjali Iyer answered |
When electric lines of force get perpendicular to equipotential surface then area vector and electric lines of force are parallel to each other.so angle between them is zero. due to that reason they get perpendicular to each other.
In bringing an electron towards another electron, the electrostatic potential energy of the system- a)becomes zero
- b)decreases
- c)remains same
- d)increases
Correct answer is option 'D'. Can you explain this answer?
In bringing an electron towards another electron, the electrostatic potential energy of the system
a)
becomes zero
b)
decreases
c)
remains same
d)
increases
|
|
Nandini Patel answered |
The electron has negative charge. When an electron is bringing towards another electron, then due to same negative charge repulsive force is produced between them. So, to bring them closer a work is done against this repulsive force. This work is stored in the form of electrostatic potential energy. Thus, electrostatic potential energy of system increases.
Alternative: Electrostatic potential energy of system of two electrons
U=[1/4πε0][(−e)(−e)/r] = [1/4πε0](e^2/r)
Thus, as r decreases, potential energy U increases.
If a unit charge is taken from one part to another part over an equipotential surface, then what is the change in electrostatic potential energy of the charge?- a)10 J
- b)100 J
- c)1 J
- d)0 J
Correct answer is option 'D'. Can you explain this answer?
If a unit charge is taken from one part to another part over an equipotential surface, then what is the change in electrostatic potential energy of the charge?
a)
10 J
b)
100 J
c)
1 J
d)
0 J

|
Utsav Srivastava answered |
Equipotential surface means the potential on every. point on that surface is constant. it means the change in potential on equipotential surface is zero we know that... ( electrostatic potential energy = change in potential × charge.)... ... according to this electrostatic potential energy is zero
A hollow metal sphere of radius 20 cm is charged such that the potential on its surface is 120 Volt. The potential at the centre of the sphere is- a)80 V
- b)6 V
- c)120 V
- d)Zero
Correct answer is 'C'. Can you explain this answer?
A hollow metal sphere of radius 20 cm is charged such that the potential on its surface is 120 Volt. The potential at the centre of the sphere is
a)
80 V
b)
6 V
c)
120 V
d)
Zero
|
|
Anjana Sharma answered |
Potential inside the charged sphere is constant and equal to potential on the surface. Hence the potential at the centre of the sphere is 120 V.
The potential energy of a system containing only one point charge is
- a)Zero
- b)Infinity
- c)Non zero finite
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
The potential energy of a system containing only one point charge is
a)
Zero
b)
Infinity
c)
Non zero finite
d)
None of the above
|
|
Manoj Chauhan answered |
Explanation:
Potential energy is defined as the work done by an external force in bringing a system from infinity to its position. Hence, the potential energy of a system containing only one point charge is given by -
U = qV
where, q is the charge of the point charge and V is the potential at its position.
Now, let's consider the two cases -
Case 1: When the point charge is at infinity
At infinity, the potential is zero as the electric field due to a point charge decreases as we move away from it. Hence, the potential energy of the system containing only one point charge at infinity is zero.
U = qV = q x 0 = 0
Case 2: When the point charge is at a finite distance from infinity
In this case, the potential energy of the system will be non-zero finite as the potential at the position of the point charge will be non-zero.
U = qV ≠ 0
Conclusion:
Hence, the correct answer is option 'A' i.e. zero as the potential energy of a system containing only one point charge is zero when the point charge is at infinity.
Potential energy is defined as the work done by an external force in bringing a system from infinity to its position. Hence, the potential energy of a system containing only one point charge is given by -
U = qV
where, q is the charge of the point charge and V is the potential at its position.
Now, let's consider the two cases -
Case 1: When the point charge is at infinity
At infinity, the potential is zero as the electric field due to a point charge decreases as we move away from it. Hence, the potential energy of the system containing only one point charge at infinity is zero.
U = qV = q x 0 = 0
Case 2: When the point charge is at a finite distance from infinity
In this case, the potential energy of the system will be non-zero finite as the potential at the position of the point charge will be non-zero.
U = qV ≠ 0
Conclusion:
Hence, the correct answer is option 'A' i.e. zero as the potential energy of a system containing only one point charge is zero when the point charge is at infinity.
Negative mutual potential energy corresponds to attraction between two charges- a)False
- b)True
- c)Can’t predict
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Negative mutual potential energy corresponds to attraction between two charges
a)
False
b)
True
c)
Can’t predict
d)
None of the above

|
Soham Rastogi answered |
The formula for electric potential energy of system of 2 charges is (kq1q2)/r, if the result comes out to be negative,one of the charges has to be negative and one has to be positive, because there can be no other case in which it comes out to be negative. Since opposite charges attract each other,hence the answer is True.
A charge q = 1.0 C moves distance of 1.5 m in the direction of a uniform electric field E of magnitude 2.0 N/C. Find its change in electrostatic potential energy.- a)2 J
- b)3 J
- c)4 J
- d)1 J
Correct answer is option 'B'. Can you explain this answer?
A charge q = 1.0 C moves distance of 1.5 m in the direction of a uniform electric field E of magnitude 2.0 N/C. Find its change in electrostatic potential energy.
a)
2 J
b)
3 J
c)
4 J
d)
1 J

|
Anchal Maurya answered |
Force (F)=E•q=2×1=2,. Energy (E)=F•d=2×1.5=3 joule
Assuming ideal gas properties, which of the following occupies the most volume at 273 K and 1 atm of pressure?- a)One mole of hydrogen gas
- b)They all occupy the same volume
- c)One mole of oxygen gas
- d)One mole of nitrogen gas
Correct answer is option 'B'. Can you explain this answer?
Assuming ideal gas properties, which of the following occupies the most volume at 273 K and 1 atm of pressure?
a)
One mole of hydrogen gas
b)
They all occupy the same volume
c)
One mole of oxygen gas
d)
One mole of nitrogen gas
|
|
Grace Adams answered |
Understanding Ideal Gas Behavior
At standard temperature and pressure (STP)—273 K and 1 atm—ideal gas behavior can be analyzed using the Ideal Gas Law. The law states that:
PV = nRT
Where:
- P = Pressure
- V = Volume
- n = Number of moles
- R = Universal gas constant
- T = Temperature
Volume Occupied by Gases
According to the Ideal Gas Law, the volume occupied by a gas is directly proportional to the number of moles when temperature and pressure are constant. Therefore, one mole of any ideal gas occupies the same volume under identical conditions of temperature and pressure.
Calculation at STP
- At STP, 1 mole of an ideal gas occupies approximately 22.4 liters.
- This is true for any ideal gas, regardless of its molecular weight or type (H2, O2, N2, etc.).
Conclusion
Thus, the correct answer to the question is:
- They all occupy the same volume.
This means that:
- One mole of hydrogen gas occupies 22.4 liters.
- One mole of oxygen gas occupies 22.4 liters.
- One mole of nitrogen gas occupies 22.4 liters.
Since they all have the same number of moles (1 mole), and the conditions are the same (273 K and 1 atm), all gases will occupy the same volume, confirming that option "B" is correct.
At standard temperature and pressure (STP)—273 K and 1 atm—ideal gas behavior can be analyzed using the Ideal Gas Law. The law states that:
PV = nRT
Where:
- P = Pressure
- V = Volume
- n = Number of moles
- R = Universal gas constant
- T = Temperature
Volume Occupied by Gases
According to the Ideal Gas Law, the volume occupied by a gas is directly proportional to the number of moles when temperature and pressure are constant. Therefore, one mole of any ideal gas occupies the same volume under identical conditions of temperature and pressure.
Calculation at STP
- At STP, 1 mole of an ideal gas occupies approximately 22.4 liters.
- This is true for any ideal gas, regardless of its molecular weight or type (H2, O2, N2, etc.).
Conclusion
Thus, the correct answer to the question is:
- They all occupy the same volume.
This means that:
- One mole of hydrogen gas occupies 22.4 liters.
- One mole of oxygen gas occupies 22.4 liters.
- One mole of nitrogen gas occupies 22.4 liters.
Since they all have the same number of moles (1 mole), and the conditions are the same (273 K and 1 atm), all gases will occupy the same volume, confirming that option "B" is correct.
Equal charges are given to two spheres of different radii. The potential will- a)be equal on both the spheres
- b)be more on the smaller sphere
- c)be more on the bigger sphere
- d)depend on the material of the sphere
Correct answer is option 'B'. Can you explain this answer?
Equal charges are given to two spheres of different radii. The potential will
a)
be equal on both the spheres
b)
be more on the smaller sphere
c)
be more on the bigger sphere
d)
depend on the material of the sphere
|
|
Rajesh Gupta answered |
When equal charges are given to two spheres of different radii, the potential will be more or the smaller sphere as per the equation, Potential = Charge / Radius.
Since potential is inversely proportional to radius, the smaller radius will have higher potential and vice versa.
Since potential is inversely proportional to radius, the smaller radius will have higher potential and vice versa.
It requires 4 J of work to move a charge of 20 C from point A to point B, separated by a distance of 0.2 cm. The potential difference between A and B in volts- a)0.2
- b)16
- c)5
- d)80
Correct answer is 'A'. Can you explain this answer?
It requires 4 J of work to move a charge of 20 C from point A to point B, separated by a distance of 0.2 cm. The potential difference between A and B in volts
a)
0.2
b)
16
c)
5
d)
80

|
Ishani Yadav answered |
Potential difference between two points is given by
Va - Vb = W/q0
Work, W = 2 J
Charge, q0 = 20 C
Potential difference = 2/20 = 0.1 V
The correct option is C.
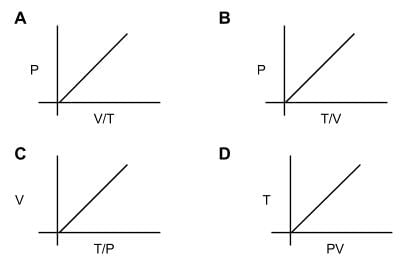
How many diagrams showing relationships between pressure, volume, and temperature below is/are incorrect for ideal gases?
- a)Only one
- b)Only three
- c)Only two
- d)None
Correct answer is option 'A'. Can you explain this answer?
How many diagrams showing relationships between pressure, volume, and temperature below is/are incorrect for ideal gases?


a)
Only one
b)
Only three
c)
Only two
d)
None
|
|
Ayesha Joshi answered |
- Although the graphs may look daunting, all of the relationships can be derived using the ideal gas law, PV=nRT.
- Just rearrange the variables in the gas law so we can see if there is a positive correlation (on different sides of the equal sign) or a negative correlation (on the same side).
- For A: P⋅V/T = nR, so this will be a negative correlation. A is false. For B: P = nR⋅T/V. B is true. For C: V = nR⋅T/P. C is true. For D: PV = nR⋅T. D is true.
- Only one diagram shows an incorrect relationship for ideal gases.
In a balloon of total pressure 6 atm there is a gaseous composition of 44 grams of carbon dioxide 16 grams of by oxygen and 7 grams of nitrogen, what is the ratio of nitrogen partial pressure do the total pressure in the balloon?- a)0.25
- b)0.5
- c)0.75
- d)1
Correct answer is option 'A'. Can you explain this answer?
In a balloon of total pressure 6 atm there is a gaseous composition of 44 grams of carbon dioxide 16 grams of by oxygen and 7 grams of nitrogen, what is the ratio of nitrogen partial pressure do the total pressure in the balloon?
a)
0.25
b)
0.5
c)
0.75
d)
1

|
Tanishq Goyal answered |
To find the ratio of nitrogen partial pressure to the total pressure in the balloon, we need to calculate the partial pressure of nitrogen and divide it by the total pressure.
First, we need to calculate the number of moles of each gas present in the balloon:
- Moles of carbon dioxide (CO2) = mass of CO2 / molar mass of CO2
= 44 g / 44 g/mol
= 1 mole of CO2
- Moles of oxygen (O2) = mass of O2 / molar mass of O2
= 16 g / 32 g/mol
= 0.5 moles of O2
- Moles of nitrogen (N2) = mass of N2 / molar mass of N2
= 7 g / 28 g/mol
= 0.25 moles of N2
Next, we need to calculate the partial pressure of each gas using the ideal gas law equation:
PV = nRT
- For carbon dioxide:
Partial pressure of CO2 = (moles of CO2 * gas constant * temperature) / volume
= (1 mole * 0.0821 atm/mol·K * T) / V
- For oxygen:
Partial pressure of O2 = (moles of O2 * gas constant * temperature) / volume
= (0.5 moles * 0.0821 atm/mol·K * T) / V
- For nitrogen:
Partial pressure of N2 = (moles of N2 * gas constant * temperature) / volume
= (0.25 moles * 0.0821 atm/mol·K * T) / V
Since we are looking for the ratio of nitrogen partial pressure to the total pressure, we can write:
Ratio = Partial pressure of N2 / Total pressure
The total pressure is given as 6 atm. Therefore, the ratio of nitrogen partial pressure to the total pressure is:
Ratio = [(0.25 moles * 0.0821 atm/mol·K * T) / V] / 6 atm
Simplifying, we can cancel out the units and rearrange the equation:
Ratio = (0.25 * 0.0821 * T) / (6 * V)
Since the question only asks for the ratio, the specific values of temperature and volume are not necessary for the calculation. So, we can conclude that the ratio of nitrogen partial pressure to the total pressure in the balloon is 0.25, which corresponds to option A.
First, we need to calculate the number of moles of each gas present in the balloon:
- Moles of carbon dioxide (CO2) = mass of CO2 / molar mass of CO2
= 44 g / 44 g/mol
= 1 mole of CO2
- Moles of oxygen (O2) = mass of O2 / molar mass of O2
= 16 g / 32 g/mol
= 0.5 moles of O2
- Moles of nitrogen (N2) = mass of N2 / molar mass of N2
= 7 g / 28 g/mol
= 0.25 moles of N2
Next, we need to calculate the partial pressure of each gas using the ideal gas law equation:
PV = nRT
- For carbon dioxide:
Partial pressure of CO2 = (moles of CO2 * gas constant * temperature) / volume
= (1 mole * 0.0821 atm/mol·K * T) / V
- For oxygen:
Partial pressure of O2 = (moles of O2 * gas constant * temperature) / volume
= (0.5 moles * 0.0821 atm/mol·K * T) / V
- For nitrogen:
Partial pressure of N2 = (moles of N2 * gas constant * temperature) / volume
= (0.25 moles * 0.0821 atm/mol·K * T) / V
Since we are looking for the ratio of nitrogen partial pressure to the total pressure, we can write:
Ratio = Partial pressure of N2 / Total pressure
The total pressure is given as 6 atm. Therefore, the ratio of nitrogen partial pressure to the total pressure is:
Ratio = [(0.25 moles * 0.0821 atm/mol·K * T) / V] / 6 atm
Simplifying, we can cancel out the units and rearrange the equation:
Ratio = (0.25 * 0.0821 * T) / (6 * V)
Since the question only asks for the ratio, the specific values of temperature and volume are not necessary for the calculation. So, we can conclude that the ratio of nitrogen partial pressure to the total pressure in the balloon is 0.25, which corresponds to option A.
A certain gas occupies 200 ml of volume at 2 bar pressure at hundred degrees Kelvin. How much volume does it occupy at 5 bar pressure and 200 degrees Kelvin?- a)200 ml
- b)160 ml
- c)240 ml
- d)320 ml
Correct answer is option 'B'. Can you explain this answer?
A certain gas occupies 200 ml of volume at 2 bar pressure at hundred degrees Kelvin. How much volume does it occupy at 5 bar pressure and 200 degrees Kelvin?
a)
200 ml
b)
160 ml
c)
240 ml
d)
320 ml
|
|
Vivek Khatri answered |
From ideal gas law, we know that P1V1/T1 = P2V2/T2. Here we take P1 as 2 bar, V1 as 200 ml, T1 as hundred degrees Kelvin, P2 as 5 bar and T2 as 200 degrees Kelvin, so by substituting the above values 200 x 2/100 = 5 x V2/200; V2 = 160 ml.
If the partial pressure of oxygen is given by three bar and the partial pressure of the other gas is four bar, then what is a total pressure that is exerted?- a)7 bar
- b)3 bar
- c)4 bar
- d)1 bar
Correct answer is option 'A'. Can you explain this answer?
If the partial pressure of oxygen is given by three bar and the partial pressure of the other gas is four bar, then what is a total pressure that is exerted?
a)
7 bar
b)
3 bar
c)
4 bar
d)
1 bar

|
Ishani Dasgupta answered |
Partial Pressure of Oxygen:
The partial pressure of oxygen is given as three bar. This means that the pressure exerted by oxygen alone is three bar.
Partial Pressure of other gas:
The partial pressure of the other gas is given as four bar. This means that the pressure exerted by this gas alone is four bar.
Total Pressure:
The total pressure is the sum of the partial pressures of all the gases present in the system. In this case, the total pressure is the sum of the partial pressure of oxygen and the partial pressure of the other gas.
Total Pressure = Partial Pressure of Oxygen + Partial Pressure of other gas
Total Pressure = 3 bar + 4 bar
Total Pressure = 7 bar
So, the total pressure exerted by both gases is seven bar.
Answer:
Therefore, the correct answer is option A) 7 bar.
The partial pressure of oxygen is given as three bar. This means that the pressure exerted by oxygen alone is three bar.
Partial Pressure of other gas:
The partial pressure of the other gas is given as four bar. This means that the pressure exerted by this gas alone is four bar.
Total Pressure:
The total pressure is the sum of the partial pressures of all the gases present in the system. In this case, the total pressure is the sum of the partial pressure of oxygen and the partial pressure of the other gas.
Total Pressure = Partial Pressure of Oxygen + Partial Pressure of other gas
Total Pressure = 3 bar + 4 bar
Total Pressure = 7 bar
So, the total pressure exerted by both gases is seven bar.
Answer:
Therefore, the correct answer is option A) 7 bar.
If the pressure of dry gas is given by X and the total pressure is given by X + 3, then what is aqueous tension?- a)2
- b)X
- c)X + 2
- d)3
Correct answer is option 'D'. Can you explain this answer?
If the pressure of dry gas is given by X and the total pressure is given by X + 3, then what is aqueous tension?
a)
2
b)
X
c)
X + 2
d)
3

|
Niharika Kulkarni answered |
Pressure of dry gas (X) and total pressure (X + 3)
Aqueous tension = ?
Given:
The pressure of dry gas is X.
The total pressure is X + 3.
To find:
The aqueous tension.
Answer and Explanation:
Aqueous tension is the partial pressure of water vapor in a mixture of gases. It can be calculated by subtracting the pressure of dry gas from the total pressure.
Aqueous tension = Total pressure - Pressure of dry gas
In this case, the total pressure is X + 3 and the pressure of dry gas is X. Therefore,
Aqueous tension = (X + 3) - X
Simplifying the expression, we get:
Aqueous tension = 3
Therefore, the correct answer is option D, 3.
Aqueous tension = ?
Given:
The pressure of dry gas is X.
The total pressure is X + 3.
To find:
The aqueous tension.
Answer and Explanation:
Aqueous tension is the partial pressure of water vapor in a mixture of gases. It can be calculated by subtracting the pressure of dry gas from the total pressure.
Aqueous tension = Total pressure - Pressure of dry gas
In this case, the total pressure is X + 3 and the pressure of dry gas is X. Therefore,
Aqueous tension = (X + 3) - X
Simplifying the expression, we get:
Aqueous tension = 3
Therefore, the correct answer is option D, 3.
The work done in bringing a unit positive charge from infinite distance to a point at distance x from a positive charge Q is W. Then the potential ϕ at that point is- a)WQ/x
- b)W
- c)W/x
- d)WQ
Correct answer is option 'B'. Can you explain this answer?
The work done in bringing a unit positive charge from infinite distance to a point at distance x from a positive charge Q is W. Then the potential ϕ at that point is
a)
WQ/x
b)
W
c)
W/x
d)
WQ
|
|
Dev Patel answered |
Electric potential at a point in an electric field is defined as the work done in bringing a unit positive charge from infinity to that point.
Or, V = W/q
Since charge is of magnitude unity, Hence electric potential at that point will be equal to the workdone ∴ ϕ = W
Or, V = W/q
Since charge is of magnitude unity, Hence electric potential at that point will be equal to the workdone ∴ ϕ = W
Which of the following do you think is a correct relationship between the molar mass of gas temperature and its pressure?- a)M = dRT/P
- b)M = dRT/V
- c)PV = nRT
- d)M = VRT/P
Correct answer is option 'A'. Can you explain this answer?
Which of the following do you think is a correct relationship between the molar mass of gas temperature and its pressure?
a)
M = dRT/P
b)
M = dRT/V
c)
PV = nRT
d)
M = VRT/P

|
Niharika Kulkarni answered |
The correct relationship between the molar mass of a gas, its temperature, and its pressure is given by the equation M = dRT/P, where M represents the molar mass of the gas, d is the density of the gas, R is the ideal gas constant, T is the temperature of the gas in Kelvin, and P is the pressure of the gas.
Explanation:
The molar mass of a gas is the mass of one mole of that gas. It is usually expressed in grams per mole (g/mol). The molar mass of a gas can be calculated by knowing the density of the gas, the ideal gas constant, the temperature of the gas, and the pressure of the gas.
- Equation M = dRT/P:
- M: Molar mass of the gas
- d: Density of the gas
- R: Ideal gas constant (8.314 J/(mol·K))
- T: Temperature of the gas in Kelvin
- P: Pressure of the gas
- Equation derivation:
- The ideal gas law equation, PV = nRT, relates the pressure (P), volume (V), amount of substance (n), ideal gas constant (R), and temperature (T) of a gas.
- By rearranging the ideal gas law equation, we can isolate the density (d) of the gas, which is defined as mass (m) divided by volume (V): d = m/V.
- Rewriting the equation in terms of density, we have P = (m/V)RT.
- Since the molar mass (M) is equal to the mass (m) divided by the amount of substance (n), we can substitute m/n with M in the equation: P = (M/nV)RT.
- The amount of substance (n) can be expressed as n = m/M, where m is the mass of the gas. Substituting n in the equation, we have P = (M/mV)RT.
- Finally, rearranging the equation, we get M = (dRT)/P, where d = m/V is the density of the gas.
Conclusion:
The correct relationship between the molar mass of a gas, its temperature, and its pressure is given by the equation M = dRT/P. This equation allows us to calculate the molar mass of a gas given its density, temperature, and pressure.
Explanation:
The molar mass of a gas is the mass of one mole of that gas. It is usually expressed in grams per mole (g/mol). The molar mass of a gas can be calculated by knowing the density of the gas, the ideal gas constant, the temperature of the gas, and the pressure of the gas.
- Equation M = dRT/P:
- M: Molar mass of the gas
- d: Density of the gas
- R: Ideal gas constant (8.314 J/(mol·K))
- T: Temperature of the gas in Kelvin
- P: Pressure of the gas
- Equation derivation:
- The ideal gas law equation, PV = nRT, relates the pressure (P), volume (V), amount of substance (n), ideal gas constant (R), and temperature (T) of a gas.
- By rearranging the ideal gas law equation, we can isolate the density (d) of the gas, which is defined as mass (m) divided by volume (V): d = m/V.
- Rewriting the equation in terms of density, we have P = (m/V)RT.
- Since the molar mass (M) is equal to the mass (m) divided by the amount of substance (n), we can substitute m/n with M in the equation: P = (M/nV)RT.
- The amount of substance (n) can be expressed as n = m/M, where m is the mass of the gas. Substituting n in the equation, we have P = (M/mV)RT.
- Finally, rearranging the equation, we get M = (dRT)/P, where d = m/V is the density of the gas.
Conclusion:
The correct relationship between the molar mass of a gas, its temperature, and its pressure is given by the equation M = dRT/P. This equation allows us to calculate the molar mass of a gas given its density, temperature, and pressure.
A long, hollow conducting cylinder is kept coaxially inside another long, hollow conducting cylinder of larger radius. Both the cylinders are initially electrically neutral.- a)No potential difference appears between the two cylinders when same charge density is given to both the cylinders.
- b)No potential difference appears between the two cylinders when a uniform line charge is kept along the axis of the cylinders.
- c)A potential difference appears between the two cylinders when a charge density is given to the outer cylinder.
- d)A potential difference appears between the two cylinders when a charge density is given to the inner cylinder.
Correct answer is option 'D'. Can you explain this answer?
A long, hollow conducting cylinder is kept coaxially inside another long, hollow conducting cylinder of larger radius. Both the cylinders are initially electrically neutral.
a)
No potential difference appears between the two cylinders when same charge density is given to both the cylinders.
b)
No potential difference appears between the two cylinders when a uniform line charge is kept along the axis of the cylinders.
c)
A potential difference appears between the two cylinders when a charge density is given to the outer cylinder.
d)
A potential difference appears between the two cylinders when a charge density is given to the inner cylinder.

|
Top Rankers answered |
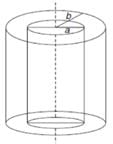
Let λ be the charge density on the inner cylinder.
For a < r < b, we get
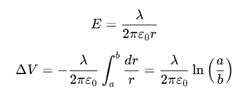
Hence, a potential difference appears between the two cylinders when a charge density is given to the inner cylinder.
Assume an experiment vessel which is well fitted with a piston. The airtight piston has negligible mass and can move up and down freely. There is 1 mol of an ideal gas contained within the vessel under the piston at pressure of 100 kPa. The piston rests at 10 cm from the base of the vessel. The pressure in the vessel increases to 200 kPa as force is applied to the piston. What would be the new resting position of the piston from the base of the vessel? Assume the temperature is held constant at 300 K throughout the experiment.- a)5 cm
- b)2 cm
- c)1 cm
- d)8 cm
Correct answer is option 'A'. Can you explain this answer?
Assume an experiment vessel which is well fitted with a piston. The airtight piston has negligible mass and can move up and down freely. There is 1 mol of an ideal gas contained within the vessel under the piston at pressure of 100 kPa. The piston rests at 10 cm from the base of the vessel. The pressure in the vessel increases to 200 kPa as force is applied to the piston. What would be the new resting position of the piston from the base of the vessel? Assume the temperature is held constant at 300 K throughout the experiment.
a)
5 cm
b)
2 cm
c)
1 cm
d)
8 cm
|
|
Ayesha Joshi answered |
- Try using Boyle's Law for this question.
- P1V1 = P2V2 , then V2 = P1V1/P2. It's important to realize that to find the volume occupied by the gas in this experiment vessel, we would use A (area of the piston) multiplied by h (the height of the piston). We then would have Ah2 = P1Ah1/P2. The surface areas actually cancel out, and you will have h2 = P1h1/P2.
- Substituting in the values, you will get the new resting position as 5 cm.
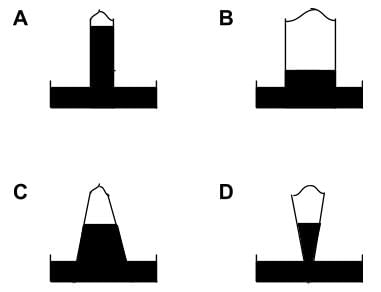
Shown below are four mercury barometers of the same height (all four barometer tubes measure one meter from the tube opening to rounded top). Which barometer shows the greatest external pressure?
- a)Barometer C
- b)Barometer D
- c)Barometer A
- d)Barometer B
Correct answer is option 'C'. Can you explain this answer?
Shown below are four mercury barometers of the same height (all four barometer tubes measure one meter from the tube opening to rounded top). Which barometer shows the greatest external pressure?


a)
Barometer C
b)
Barometer D
c)
Barometer A
d)
Barometer B

|
Orion Classes answered |
- Consider the difference in shapes between the different barometers.
- Given that the force acting on the fluid in the barometer is F = mg. We can derive it thus: m = density⋅volume, so F = density⋅volume⋅g. Volume = area⋅height, so F=density⋅area⋅height⋅g. We know that F/area = pressure, so we can say that the change in pressure = the change in height⋅density⋅g.
Which of the following assumptions is NOT part of the Kinetic Molecular Theory of Gases?- a)Gas molecules occupy a negligible volume.
- b)Gas molecules are in constant motion.
- c)Gas molecules have strong attractive forces between them.
- d)Gas molecules undergo elastic collisions.
Correct answer is option 'C'. Can you explain this answer?
Which of the following assumptions is NOT part of the Kinetic Molecular Theory of Gases?
a)
Gas molecules occupy a negligible volume.
b)
Gas molecules are in constant motion.
c)
Gas molecules have strong attractive forces between them.
d)
Gas molecules undergo elastic collisions.
|
|
Ayesha Joshi answered |
The Kinetic Molecular Theory assumes that gas molecules do not have strong attractive forces between them. Instead, they only interact through collisions.
According to the Kinetic Molecular Theory of Gases, increasing the temperature of a gas sample at constant volume will result in:- a)An increase in the number of gas molecules.
- b)A decrease in the average kinetic energy of the gas molecules.
- c)An increase in the pressure of the gas.
- d)No change in the behavior of the gas.
Correct answer is option 'C'. Can you explain this answer?
According to the Kinetic Molecular Theory of Gases, increasing the temperature of a gas sample at constant volume will result in:
a)
An increase in the number of gas molecules.
b)
A decrease in the average kinetic energy of the gas molecules.
c)
An increase in the pressure of the gas.
d)
No change in the behavior of the gas.
|
|
Liam Johnson answered |
Understanding the Kinetic Molecular Theory of Gases
The Kinetic Molecular Theory (KMT) offers insights into the behavior of gases, particularly how temperature affects their characteristics.
Temperature and Kinetic Energy
- Kinetic Energy: According to KMT, the temperature of a gas is directly related to the average kinetic energy of its molecules.
- Effect of Temperature Increase: When the temperature of a gas increases, the average kinetic energy of the gas molecules also increases. This means that the molecules move faster.
Constant Volume Implications
- Constant Volume: If the gas is held at a constant volume, the space available for the gas molecules to move does not change.
- Resulting Pressure Increase: As the temperature rises, the faster-moving molecules collide with the walls of the container more frequently and with greater force.
Pressure Change in Gases
- Pressure and Temperature Relationship: At constant volume, increasing the temperature leads to an increase in pressure. This is described by the ideal gas law, where pressure is directly proportional to temperature (in Kelvin) when volume is constant.
Correct Answer Explanation
- Option C: Therefore, the correct answer to the question is option 'C' - an increase in the pressure of the gas.
- Other Options:
- Option 'A' is incorrect as the number of gas molecules remains constant.
- Option 'B' is incorrect since temperature increase raises the average kinetic energy, not decreases it.
- Option 'D' is also incorrect; the behavior of the gas changes with temperature.
In summary, increasing the temperature of a gas at constant volume results in increased pressure due to heightened molecular activity.
The Kinetic Molecular Theory (KMT) offers insights into the behavior of gases, particularly how temperature affects their characteristics.
Temperature and Kinetic Energy
- Kinetic Energy: According to KMT, the temperature of a gas is directly related to the average kinetic energy of its molecules.
- Effect of Temperature Increase: When the temperature of a gas increases, the average kinetic energy of the gas molecules also increases. This means that the molecules move faster.
Constant Volume Implications
- Constant Volume: If the gas is held at a constant volume, the space available for the gas molecules to move does not change.
- Resulting Pressure Increase: As the temperature rises, the faster-moving molecules collide with the walls of the container more frequently and with greater force.
Pressure Change in Gases
- Pressure and Temperature Relationship: At constant volume, increasing the temperature leads to an increase in pressure. This is described by the ideal gas law, where pressure is directly proportional to temperature (in Kelvin) when volume is constant.
Correct Answer Explanation
- Option C: Therefore, the correct answer to the question is option 'C' - an increase in the pressure of the gas.
- Other Options:
- Option 'A' is incorrect as the number of gas molecules remains constant.
- Option 'B' is incorrect since temperature increase raises the average kinetic energy, not decreases it.
- Option 'D' is also incorrect; the behavior of the gas changes with temperature.
In summary, increasing the temperature of a gas at constant volume results in increased pressure due to heightened molecular activity.
Consider a gas of n moles at a pressure of P and a temperature of T in Celsius, what would be its volume?- a)nR(T + 273)/p
- b)nRT/p
- c)nR(T – 273)/p
- d)R(T + 273)/p
Correct answer is option 'A'. Can you explain this answer?
Consider a gas of n moles at a pressure of P and a temperature of T in Celsius, what would be its volume?
a)
nR(T + 273)/p
b)
nRT/p
c)
nR(T – 273)/p
d)
R(T + 273)/p

|
Dipanjan Sharma answered |
The correct answer is b) nRT/P. This follows from the ideal gas law equation:
PV = nRT
where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin. To convert Celsius to Kelvin, we add 273 to the temperature. Rearranging the equation, we get:
V = nRT/P
Substituting the given values, we get:
V = nR(T+273)/P
PV = nRT
where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin. To convert Celsius to Kelvin, we add 273 to the temperature. Rearranging the equation, we get:
V = nRT/P
Substituting the given values, we get:
V = nR(T+273)/P
The potential at a point due to a charge of 5×10−7C located 10cm10cm away is- a)3.5 × 105V
- b)3.5 × 104V
- c)4.5 × 104V
- d)4.5 × 105V
Correct answer is option 'C'. Can you explain this answer?
The potential at a point due to a charge of 5×10−7C located 10cm10cm away is
a)
3.5 × 105V
b)
3.5 × 104V
c)
4.5 × 104V
d)
4.5 × 105V
|
|
Dev Patel answered |
Here, q = 5 × 10(−7) C, r = 10 cm = 0.1 m
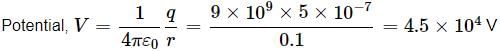
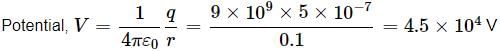
Two points A and B are located in diametrically opposite directions of a point charge of +2μC at distances 2m and 1m respectively from it. The potential difference between A and B is- a)3 × 103V
- b)6 × 104V
- c)−9 × 103V
- d)−3 × 103V
Correct answer is option 'C'. Can you explain this answer?
Two points A and B are located in diametrically opposite directions of a point charge of +2μC at distances 2m and 1m respectively from it. The potential difference between A and B is
a)
3 × 103V
b)
6 × 104V
c)
−9 × 103V
d)
−3 × 103V
|
|
Priya Menon answered |
Here, q = 2 μC = 2 × 10−6 C,
rA = 2m, rB = 1m
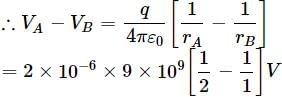
= −9 × 103 V
rA = 2m, rB = 1m
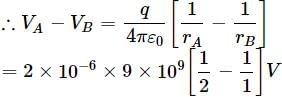
= −9 × 103 V
Which of the following statement is not true?- a)Electrostatic force is a conservative force.
- b)Potential at a point is the work done per unit charge in bringing a charge from any point to infinity.
- c)Electrostatic force is non-conservative.
- d)Potential is the product of charge and work.
Correct answer is option 'B'. Can you explain this answer?
Which of the following statement is not true?
a)
Electrostatic force is a conservative force.
b)
Potential at a point is the work done per unit charge in bringing a charge from any point to infinity.
c)
Electrostatic force is non-conservative.
d)
Potential is the product of charge and work.
|
|
Mira Joshi answered |
Work done by the electrostatic force is independent of the path followed by it, and it depends only on the initial and final positions. For example, work done in moving a unit positive charge in a closed loop of an electric field is zero.
Who gave the law regarding the partial pressure?- a)Charles
- b)Dalton
- c)Lussac
- d)Thomas
Correct answer is option 'B'. Can you explain this answer?
Who gave the law regarding the partial pressure?
a)
Charles
b)
Dalton
c)
Lussac
d)
Thomas

|
Hrishikesh Verma answered |
Dalton gave the law regarding the partial pressure.
Partial pressure refers to the pressure exerted by an individual component of a gas mixture. It is the pressure that the component would exert if it occupied the same volume alone at the same temperature. Dalton's law of partial pressures states that the total pressure exerted by a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases.
Explanation:
Dalton's law of partial pressures is based on the kinetic theory of gases and is named after the English chemist and physicist John Dalton. He formulated this law in 1801.
The law states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases. Mathematically, it can be expressed as:
P_total = P_1 + P_2 + P_3 + ... + P_n
where P_total is the total pressure of the gas mixture, and P_1, P_2, P_3, ..., P_n are the partial pressures of the individual gases in the mixture.
Example:
For example, let's consider a mixture of oxygen (O2) and nitrogen (N2) gases. If the partial pressure of oxygen is 200 kPa and the partial pressure of nitrogen is 300 kPa, then the total pressure of the gas mixture would be 500 kPa according to Dalton's law of partial pressures.
Significance:
Dalton's law of partial pressures is significant in many areas of chemistry and physics. It is particularly important in the study of gas mixtures and their behavior. The law helps in understanding gas behavior, calculating gas concentrations, and predicting the behavior of gases in various conditions.
Conclusion:
In conclusion, Dalton's law of partial pressures, formulated by Dalton, states that the total pressure exerted by a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases. This law is crucial in understanding gas behavior and has applications in various fields of science and technology.
Partial pressure refers to the pressure exerted by an individual component of a gas mixture. It is the pressure that the component would exert if it occupied the same volume alone at the same temperature. Dalton's law of partial pressures states that the total pressure exerted by a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases.
Explanation:
Dalton's law of partial pressures is based on the kinetic theory of gases and is named after the English chemist and physicist John Dalton. He formulated this law in 1801.
The law states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases. Mathematically, it can be expressed as:
P_total = P_1 + P_2 + P_3 + ... + P_n
where P_total is the total pressure of the gas mixture, and P_1, P_2, P_3, ..., P_n are the partial pressures of the individual gases in the mixture.
Example:
For example, let's consider a mixture of oxygen (O2) and nitrogen (N2) gases. If the partial pressure of oxygen is 200 kPa and the partial pressure of nitrogen is 300 kPa, then the total pressure of the gas mixture would be 500 kPa according to Dalton's law of partial pressures.
Significance:
Dalton's law of partial pressures is significant in many areas of chemistry and physics. It is particularly important in the study of gas mixtures and their behavior. The law helps in understanding gas behavior, calculating gas concentrations, and predicting the behavior of gases in various conditions.
Conclusion:
In conclusion, Dalton's law of partial pressures, formulated by Dalton, states that the total pressure exerted by a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases. This law is crucial in understanding gas behavior and has applications in various fields of science and technology.
The partial pressure of a gas X is given by two bar, where is the total pressure of the gaseous mixture in a cylinder is 10 bar. What is the mole fraction of the gas X in that mixture?- a)0.5
- b)2
- c)0.2
- d)5
Correct answer is option 'C'. Can you explain this answer?
The partial pressure of a gas X is given by two bar, where is the total pressure of the gaseous mixture in a cylinder is 10 bar. What is the mole fraction of the gas X in that mixture?
a)
0.5
b)
2
c)
0.2
d)
5
|
|
Vivek Khatri answered |
The partial pressure of a gas equals the mole fraction of the gas in the gaseous mixture x the total pressure that is exerted by the gaseous mixture. So here 2 bar = molar fraction x 10 bar, we get that the mole fraction is 2/10 = 0.2.
A particle of charge q1 = 3μC is located on x-axis at the point x1 = 6 cm. A second point charge q2 = 2μC is placed on the x-axis at x2 = -4 cm. The absolute electric potential at the origin is- a)9 x 10-6 V
- b)9 x 105 V
- c)9 x 10-4 V
- d)9 x 10-2 V
Correct answer is option 'B'. Can you explain this answer?
A particle of charge q1 = 3μC is located on x-axis at the point x1 = 6 cm. A second point charge q2 = 2μC is placed on the x-axis at x2 = -4 cm. The absolute electric potential at the origin is
a)
9 x 10-6 V
b)
9 x 105 V
c)
9 x 10-4 V
d)
9 x 10-2 V

|
Lead Academy answered |
The absolute electric potential at the origin due to two point charges can be calculated using the formula:
- V = k (q1/r1 + q2/r2)
Where:
- V = electric potential
- k = 8.99 x 109 N m2/C2 (Coulomb's constant)
- q1 = 3 µC (charge at x1 = 6 cm)
- q2 = 2 µC (charge at x2 = -4 cm)
- r1 = 6 cm = 0.06 m (distance from origin to q1)
- r2 = 4 cm = 0.04 m (distance from origin to q2)
Substituting the values:
- V = 8.99 x 109 × (3 x 10-6/0.06 + 2 x 10-6/0.04)
Calculating each term:
- For q1: 3 x 10-6/0.06 = 5 x 10-5
- For q2: 2 x 10-6/0.04 = 5 x 10-5
Therefore:
- V = 8.99 x 109 × (5 x 10-5 + 5 x 10-5)
- V = 8.99 x 109 × 10 x 10-5 = 8.99 x 105 V
Thus, the absolute electric potential at the origin is: 9 x 105 V.
Chapter doubts & questions for Gas Phase (GC, PHY) - MCAT Chemical and Physical Foundations 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Gas Phase (GC, PHY) - MCAT Chemical and Physical Foundations in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
MCAT Chemical and Physical Foundations
336 videos|223 docs|109 tests
|