All Exams >
MCAT >
MCAT Chemical and Physical Foundations >
All Questions
All questions of Periodic Table (GC) for MCAT Exam
What is the relationship between the number of elements in each period and the number of atomic orbitals available in the energy level that is being filled?- a)Number of elements is twice the number of atomic orbitals.
- b)Number of elements is three times the number of atomic orbitals.
- c)Number of elements is half the number of atomic orbitals.
- d)Number of elements is same as the number of atomic orbitals.
Correct answer is 'A'. Can you explain this answer?
What is the relationship between the number of elements in each period and the number of atomic orbitals available in the energy level that is being filled?
a)
Number of elements is twice the number of atomic orbitals.
b)
Number of elements is three times the number of atomic orbitals.
c)
Number of elements is half the number of atomic orbitals.
d)
Number of elements is same as the number of atomic orbitals.
|
|
Krishna Iyer answered |
The number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled.
Let's understand it by taking the elements of the 4th period.
In the fourth period, there are 18 elements. The types of subshells used in the 4th period are s, p, and d.
Number of orbitals of s subshell = 1
Number of orbitals of p subshell = 3
Number of orbitals of d subshell = 5
Total number of orbitals = 9
The total number of elements is 18 i.e. twice the total number of orbitals.
In the fourth period, there are 18 elements. The types of subshells used in the 4th period are s, p, and d.
Number of orbitals of s subshell = 1
Number of orbitals of p subshell = 3
Number of orbitals of d subshell = 5
Total number of orbitals = 9
The total number of elements is 18 i.e. twice the total number of orbitals.
The lanthanide series starts with:
- a)Thorium
- b)Lutetium
- c)Lawrencium
- d)cerium
Correct answer is option 'D'. Can you explain this answer?
The lanthanide series starts with:
a)
Thorium
b)
Lutetium
c)
Lawrencium
d)
cerium
|
|
Suresh Reddy answered |
The lanthanide series starts from cerium and ends in lutetium
Elements in the same group have same:- a)Number of valence electrons
- b)Density
- c)Atomic radius
- d)Nuclear charge
Correct answer is option 'A'. Can you explain this answer?
Elements in the same group have same:
a)
Number of valence electrons
b)
Density
c)
Atomic radius
d)
Nuclear charge
|
|
Lavanya Menon answered |
- Elements in the same group have the same number of valence electrons in their outermost shell, hence, have similar properties.
- Elements in the same period don't have same number of valence electrons, hence, have different properties. But, elements in the same period have same number of shells.
The symbol for element with atomic number 111 and name Unununnium is- a)Uun
- b)Uuu
- c)UUU
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
The symbol for element with atomic number 111 and name Unununnium is
a)
Uun
b)
Uuu
c)
UUU
d)
None of the above
|
|
Gaurav Kumar answered |
- The element was to be called unununium (with the corresponding symbol of Uuu),a systematic element name as a placeholder, until the element was discovered (and the discovery then confirmed) and a permanent name was decided on.
- Although widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, the recommendations were mostly ignored among scientists in the field, who called it element 111, with the symbol of E111, (111) or even simply 111.
The first period has how many orbitals?- a)1
- b)3
- c)2
- d)4
Correct answer is option 'A'. Can you explain this answer?
The first period has how many orbitals?
a)
1
b)
3
c)
2
d)
4
|
|
Raghav Bansal answered |
1st period contains only 2 elements hydrogen and helium having atomic no. 1 and 2 respectively.
The electronic configuration of hydrogen is 1s1 while that of helium is 1s2. Both contain only s subshell. S subshell that only contains 1 orbital i.e. z.
Thus, 1st period has 1 orbital.
What is the maximum number of electrons that can be filled in 3d subshell?- a)2
- b)14
- c)6
- d)10
Correct answer is option 'D'. Can you explain this answer?
What is the maximum number of electrons that can be filled in 3d subshell?
a)
2
b)
14
c)
6
d)
10
|
|
Shreya Gupta answered |
There are 5 orbitals in d subshell and each orbital can accommodate 2 electrons. Hence maximum number of electrons that can be filled in 3d subshell is 10.
The maximum number of elements present in seventh period of the modern periodic table is:- a)32
- b)8
- c)2
- d)18
Correct answer is option 'A'. Can you explain this answer?
The maximum number of elements present in seventh period of the modern periodic table is:
a)
32
b)
8
c)
2
d)
18
|
|
Anjali Iyer answered |
The total number of electrons that can be accommodated in seventh period are 2 ( in 7s) + 14(in 5f) + 10(in 6d )+ 6(in 7p) = 32. The maximum number of elements present in it is 32.
How many orbitals are filled in second period?- a)6
- b)3
- c)1
- d)4
Correct answer is option 'D'. Can you explain this answer?
How many orbitals are filled in second period?
a)
6
b)
3
c)
1
d)
4
|
|
Lavanya Menon answered |
The 4 orbitals filled in second period are one 2s (with 2 electrons) and three 2p (with 6 electrons).
What is the principal quantum number for second period?a)1b)3c)2d)4Correct answer is option 'C'. Can you explain this answer?
|
|
Krishna Iyer answered |
Principle quantum no is defined as the no of shells an element possesses. Since every element in the 2nd period has 2 shells, therefore, principle quantum no for 2nd period is 2.
The 3d transition series starts from which atomic number?- a)19
- b)21
- c)20
- d)22
Correct answer is option 'B'. Can you explain this answer?
The 3d transition series starts from which atomic number?
a)
19
b)
21
c)
20
d)
22
|
|
Ishani Pillai answered |
The 3d transition series starts from atomic number 21, which is the element Scandium (Sc).
Explanation:
- The transition elements or transition metals are a group of elements in the periodic table that have partially filled d orbitals in their valence shells.
- The 3d transition series refers to the elements that have their d orbitals filled progressively, from scandium (Sc) to zinc (Zn), as the atomic number increases.
- Scandium has an atomic number of 21, which means it has 21 protons in its nucleus and 21 electrons surrounding it.
- Scandium is the first element in the 3d transition series and has one electron in its 3d orbital.
- The other elements in the 3d transition series are titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn).
- These elements have their d orbitals filled progressively, from 1 electron in Sc to 10 electrons in Zn.
- The 4d and 5d transition series follow the same pattern, with the d orbitals being progressively filled from the first element in each series.
Therefore, the correct answer is option B, 21.
Explanation:
- The transition elements or transition metals are a group of elements in the periodic table that have partially filled d orbitals in their valence shells.
- The 3d transition series refers to the elements that have their d orbitals filled progressively, from scandium (Sc) to zinc (Zn), as the atomic number increases.
- Scandium has an atomic number of 21, which means it has 21 protons in its nucleus and 21 electrons surrounding it.
- Scandium is the first element in the 3d transition series and has one electron in its 3d orbital.
- The other elements in the 3d transition series are titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn).
- These elements have their d orbitals filled progressively, from 1 electron in Sc to 10 electrons in Zn.
- The 4d and 5d transition series follow the same pattern, with the d orbitals being progressively filled from the first element in each series.
Therefore, the correct answer is option B, 21.
The last element of actinoid series is:- a)Lutetium
- b)Thorium
- c)Cerium
- d)Lawrencium
Correct answer is option 'D'. Can you explain this answer?
The last element of actinoid series is:
a)
Lutetium
b)
Thorium
c)
Cerium
d)
Lawrencium
|
|
Pooja Mehta answered |
The last element in the actinoid series is lawrencium, Lr. Its atomic number is 103 and its electronic configuration is[Rn]5 f146d17s2. The most common oxidation state displayed by it is +3; because after losing 3 electrons it attains stable f14 configuration.
For IUPAC nomenclature of elements with atomic number greater than 100, the abbreviation used for digit zero is:- a)o
- b)MN
- c)n
- d)N
Correct answer is 'C'. Can you explain this answer?
For IUPAC nomenclature of elements with atomic number greater than 100, the abbreviation used for digit zero is:
a)
o
b)
MN
c)
n
d)
N
|
|
Mira Sharma answered |
For IUPAC nomenclature of elements with atomic number greater than 100, the abbreviation used for digit zero is n and name is nil.
The distribution of electrons into orbitals of an atom is called its:- a)Electronic configuration
- b)Atomic configuration
- c)Molecular configuration
- d)Configuration
Correct answer is option 'A'. Can you explain this answer?
The distribution of electrons into orbitals of an atom is called its:
a)
Electronic configuration
b)
Atomic configuration
c)
Molecular configuration
d)
Configuration

|
Vaishnavi Bajaj answered |
The arrangement of electrons in different shells and sub-shells is known as the electronic configuration of a particular element. The electronic configuration diagram represents an element in its ground state or stable state. There are a set of rules to remember while distribution off electrons in different orbits.
The Latin word commonly used for the digit 9 is:- a)Hex
- b)Nil
- c)Quad
- d)Enn
Correct answer is option 'D'. Can you explain this answer?
The Latin word commonly used for the digit 9 is:
a)
Hex
b)
Nil
c)
Quad
d)
Enn
|
|
Om Desai answered |
The Latin word commonly used for the digit 9 is “enn”. It is abbreviated as “e”.
Elements in the same vertical group of the Modern Periodic Table have same:- a)Number of protons
- b)Atomic number
- c)Number of electrons
- d)Electronic configuration of outermost shell
Correct answer is option 'D'. Can you explain this answer?
Elements in the same vertical group of the Modern Periodic Table have same:
a)
Number of protons
b)
Atomic number
c)
Number of electrons
d)
Electronic configuration of outermost shell
|
|
Priyanshu Intelligent answered |
For example
He li na ki rb se fariyad .
They have one Electron in last shell (I.e. same electronic configuration).
Beta Maange Car Scooter Baaap Razi .
2 electrons in last shell .
And so on ....
He li na ki rb se fariyad .
They have one Electron in last shell (I.e. same electronic configuration).
Beta Maange Car Scooter Baaap Razi .
2 electrons in last shell .
And so on ....
The electronic configuration of an element with atomic number (Z = 11) is 1s2, 2s2, 2p6, 3s1. The symbol of the element is:- a)Li
- b)Cs
- c)Rb
- d)Na
Correct answer is option 'D'. Can you explain this answer?
The electronic configuration of an element with atomic number (Z = 11) is 1s2, 2s2, 2p6, 3s1. The symbol of the element is:
a)
Li
b)
Cs
c)
Rb
d)
Na
|
|
Naina Sharma answered |
The atomic number of sodium (Na) is 11. So its electronic configuration is 1s2, 2s2, 2p6, 3s1.
The elements beyond atomic number (Z = 92) are known as:- a)carbon family
- b)oxygen family
- c)trans fermium elements
- d)Transuranium elements
Correct answer is option 'D'. Can you explain this answer?
The elements beyond atomic number (Z = 92) are known as:
a)
carbon family
b)
oxygen family
c)
trans fermium elements
d)
Transuranium elements

|
Ayush Joshi answered |
Transuranium element. The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92 (the atomic number of uranium). All of these elements are unstable and decay radioactively into other elements.
Which of the following statements accurately describes the first ionization energy data shown in the table?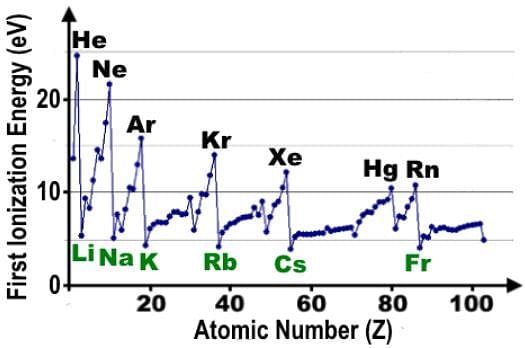
- a)Ionization energy increases as we move across a group on the periodic table.
- b)All the local peaks labelled in black correspond to the noble gases, and all the local minima or dips labelled in green correspond to the alkali metals.
- c)The exceptions to the ionization energy trend across a particular period correspond to the formation of half-filled and fully-filled orbitals.
- d)The plateaus in the data correspond to the actinide and lanthanide series, sometimes referred to as the “inner transition” metals.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements accurately describes the first ionization energy data shown in the table?

a)
Ionization energy increases as we move across a group on the periodic table.
b)
All the local peaks labelled in black correspond to the noble gases, and all the local minima or dips labelled in green correspond to the alkali metals.
c)
The exceptions to the ionization energy trend across a particular period correspond to the formation of half-filled and fully-filled orbitals.
d)
The plateaus in the data correspond to the actinide and lanthanide series, sometimes referred to as the “inner transition” metals.

|
Orion Classes answered |
Ionization energy is the energy needed to remove an electron from a neutral atom in the gaseous state and is considered endothermic.
As a general trend, ionization energy increases moving across a period on the periodic table, but the data seems to indicate there are dips along the way.
The local peaks labelled in black correspond to all the noble gases plus Hg or mercury, and the local minima correspond to the alkali metals.
The plateaus in the data correspond to the transition metals and the inner transition metals. Between K and Kr, there is one set of transition metals, and another between Rb and Xe.
The exceptions to the trend or the small dips correspond to half-filled and fully-filled orbitals. Between Li and Ne, there are two dips which correspond to B and O.
What comes prior to each is an element with half-filled or fully-filled orbitals. Be comes before B, and because of the stability of the fully-filled s orbital, it takes more energy to rip off an electron from the neutral atom. The same applies to N with half-filled p orbitals.
_______ is also known as ‘Oil of Vitriol’.- a)Nitric acid
- b)Sulphuric acid
- c)Hydrochloric acid
- d)Citric acid
Correct answer is option 'B'. Can you explain this answer?
_______ is also known as ‘Oil of Vitriol’.
a)
Nitric acid
b)
Sulphuric acid
c)
Hydrochloric acid
d)
Citric acid
|
|
Ipsita Menon answered |
There is no specific word or term provided in the question. Please provide more information or context for a more accurate answer.
Consider the following statements with respect to noble metals:
1. Noble metals are found in pure form in nature.
2. Uranium and lead are examples of noble metal.- a)Brass is also noble metal
- b)1 only
- c)2 only
- d)Both 1 and 2
Correct answer is option 'B'. Can you explain this answer?
Consider the following statements with respect to noble metals:
1. Noble metals are found in pure form in nature.
2. Uranium and lead are examples of noble metal.
1. Noble metals are found in pure form in nature.
2. Uranium and lead are examples of noble metal.
a)
Brass is also noble metal
b)
1 only
c)
2 only
d)
Both 1 and 2
|
|
Anshika Shah answered |
Noble Metals
Noble metals are a group of metals that have certain distinct properties, making them highly valuable and sought after. These metals are known for their resistance to corrosion and oxidation, as well as their ability to withstand high temperatures. Some common examples of noble metals include gold, silver, platinum, and palladium.
Statement 1: Noble metals are found in pure form in nature.
This statement is true. Noble metals are often found in nature in their pure form, which means they are not combined with other elements. This is due to their low reactivity and resistance to corrosion. For example, gold nuggets are often found in rivers and streams in their pure form, without the need for extensive refining processes. Similarly, platinum and palladium are also found in nature in their elemental state.
Statement 2: Uranium and lead are examples of noble metal.
This statement is false. Uranium and lead are not considered noble metals. Uranium is a radioactive metal that is highly reactive and undergoes radioactive decay, while lead is a heavy metal that is more prone to corrosion and oxidation. Noble metals, on the other hand, are characterized by their low reactivity and resistance to corrosion.
Summary:
- Noble metals are highly valued for their resistance to corrosion and oxidation, as well as their ability to withstand high temperatures.
- Noble metals are often found in nature in their pure form, without the need for extensive refining processes.
- Examples of noble metals include gold, silver, platinum, and palladium.
- Uranium and lead are not considered noble metals due to their high reactivity and susceptibility to corrosion.
Noble metals are a group of metals that have certain distinct properties, making them highly valuable and sought after. These metals are known for their resistance to corrosion and oxidation, as well as their ability to withstand high temperatures. Some common examples of noble metals include gold, silver, platinum, and palladium.
Statement 1: Noble metals are found in pure form in nature.
This statement is true. Noble metals are often found in nature in their pure form, which means they are not combined with other elements. This is due to their low reactivity and resistance to corrosion. For example, gold nuggets are often found in rivers and streams in their pure form, without the need for extensive refining processes. Similarly, platinum and palladium are also found in nature in their elemental state.
Statement 2: Uranium and lead are examples of noble metal.
This statement is false. Uranium and lead are not considered noble metals. Uranium is a radioactive metal that is highly reactive and undergoes radioactive decay, while lead is a heavy metal that is more prone to corrosion and oxidation. Noble metals, on the other hand, are characterized by their low reactivity and resistance to corrosion.
Summary:
- Noble metals are highly valued for their resistance to corrosion and oxidation, as well as their ability to withstand high temperatures.
- Noble metals are often found in nature in their pure form, without the need for extensive refining processes.
- Examples of noble metals include gold, silver, platinum, and palladium.
- Uranium and lead are not considered noble metals due to their high reactivity and susceptibility to corrosion.
Which of the following statements accurately describes the electron affinity data in the chart below?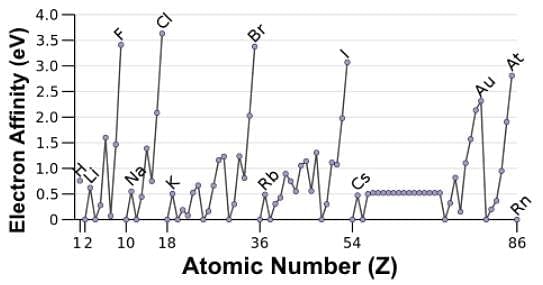
- a)The electron affinity values are smaller especially for the nonmetals in Period II because the repulsion between the electron being added and the electron already present is greatest for such atoms.
- b)The maxima correspond to the noble gas configurations because they have the greatest effective nuclear charge in a particular group.
- c)Electron affinity decreases as you move down the group due to increasing electron shells.
- d)Each of the minima correspond to elements with fully-filled orbitals since it would be energetically unfavorable to add an electron to such configurations.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements accurately describes the electron affinity data in the chart below?

a)
The electron affinity values are smaller especially for the nonmetals in Period II because the repulsion between the electron being added and the electron already present is greatest for such atoms.
b)
The maxima correspond to the noble gas configurations because they have the greatest effective nuclear charge in a particular group.
c)
Electron affinity decreases as you move down the group due to increasing electron shells.
d)
Each of the minima correspond to elements with fully-filled orbitals since it would be energetically unfavorable to add an electron to such configurations.

|
Orion Classes answered |
The minima correspond to the fully-filled s and p orbitals and some of the half-filled p and d orbitals. The stability of such configurations makes adding an electron energetically unfavorable where some elements have electron affinities (EA) that are endothermic.
There is no clear trend moving down a group due to increasing electron shells. Analyzing the data for the halides, for instance, which are the peaks, will reveal that fluorine has a lower EA than chlorine, which has the highest EA out of the group.
The maxima correspond to the halides since by acquiring one more electron they achieve a complete octet or the noble gas configuration.
The values are smaller for the nonmetals in Period II, i.e. O and F, because the repulsion between the electron being added and the electrons already present is greatest. Upon adding an electron to the neutral atom, the repulsion is more greatly felt due to their small size, and hence less energy is released.
Which of the following statements accurately describes the different classifications of elements?- a)Noble metals like gold, silver, and platinum are resistant to corrosion due to their fully-filled d-bands.
- b)The alkali earth metals react with water to form hydrogen gas as well as the metal oxides in aqueous solution.
- c)The alkali metals have distinctive flame colors because of their easily excited d electrons.
- d)All the halogens react with hydrogen to form hydrogen halides, which when mixed with water form strong acids.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements accurately describes the different classifications of elements?
a)
Noble metals like gold, silver, and platinum are resistant to corrosion due to their fully-filled d-bands.
b)
The alkali earth metals react with water to form hydrogen gas as well as the metal oxides in aqueous solution.
c)
The alkali metals have distinctive flame colors because of their easily excited d electrons.
d)
All the halogens react with hydrogen to form hydrogen halides, which when mixed with water form strong acids.
|
|
Ayesha Joshi answered |
The common classifications of elements are the alkali metals (Group I), alkali earth metals (Group II), pnictogens (Group V), chalcogens (Group VI), halogens (Group VII), and noble gases (group VIII).
As for alkali metals, they have distinctive flame colors because of their easily excited electron in the s shell. Not all alkali metals even have d electrons.
As for alkali earth metals, they react with water to form hydrogen gas and the respective alkaline hydroxides in aqueous solution. Metal oxides became hydroxides in water.
All the halogens do react with hydrogen gas to form hydrogen halides, i.e. HF, HCl, HBr, HI, HAt. Not all of them form strong acids since HF is considered a weak acid because of the high electronegativity of fluorine. HAt would be the strongest acid but it readily decomposes back to hydrogen gas and astatine, and astatine has a short half-life.
The transition metals include the designation of noble metals, which comprises ruthenium, rhodium, palladium, silver, osmium, iridium, platinum, and gold. The noble metals resist corrosion because their d orbitals are fully-filled.
The element Y having atomic number 12 is- a)A metal
- b)A metalloid
- c)Non-metal
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
The element Y having atomic number 12 is
a)
A metal
b)
A metalloid
c)
Non-metal
d)
None of the above
|
|
Shruti Rane answered |
The element Y having atomic number 12
The periodic table is a tabular arrangement of chemical elements that are organized based on their atomic number, electron configuration, and recurring chemical properties. Elements are classified into different groups or categories based on their properties. One such classification is based on the metallic and non-metallic nature of the elements.
The element Y, which has an atomic number of 12, is classified as a metal. Metals are elements that generally have a shiny appearance, are good conductors of heat and electricity, and are malleable and ductile. They also tend to form positive ions by losing electrons.
Metals possess several characteristic properties that differentiate them from non-metals. Some key properties of metals include:
Metallic luster: Metals have a characteristic shiny or metallic luster due to the presence of free electrons that can reflect light.
Conductivity: Metals are excellent conductors of heat and electricity. This is because they have a delocalized sea of electrons that can easily move and transfer energy.
Malleability and ductility: Metals can be easily hammered into thin sheets (malleability) and drawn into wires (ductility) without breaking. This is due to the ability of metal atoms to roll over each other without disturbing the metallic bond.
High melting and boiling points: Metals generally have high melting and boiling points due to the strong metallic bonds between atoms. These bonds require a significant amount of energy to break.
Reactivity: Metals tend to lose electrons and form positive ions (cations) when they undergo chemical reactions. This makes them more likely to react with non-metals and form compounds.
Based on the given information, the element Y with atomic number 12 is classified as a metal. It exhibits the characteristic properties of metals, such as metallic luster, conductivity, malleability, ductility, and reactivity.
Introduction
The periodic table is a tabular arrangement of chemical elements that are organized based on their atomic number, electron configuration, and recurring chemical properties. Elements are classified into different groups or categories based on their properties. One such classification is based on the metallic and non-metallic nature of the elements.
Explanation
The element Y, which has an atomic number of 12, is classified as a metal. Metals are elements that generally have a shiny appearance, are good conductors of heat and electricity, and are malleable and ductile. They also tend to form positive ions by losing electrons.
Properties of metals
Metals possess several characteristic properties that differentiate them from non-metals. Some key properties of metals include:
Metallic luster: Metals have a characteristic shiny or metallic luster due to the presence of free electrons that can reflect light.
Conductivity: Metals are excellent conductors of heat and electricity. This is because they have a delocalized sea of electrons that can easily move and transfer energy.
Malleability and ductility: Metals can be easily hammered into thin sheets (malleability) and drawn into wires (ductility) without breaking. This is due to the ability of metal atoms to roll over each other without disturbing the metallic bond.
High melting and boiling points: Metals generally have high melting and boiling points due to the strong metallic bonds between atoms. These bonds require a significant amount of energy to break.
Reactivity: Metals tend to lose electrons and form positive ions (cations) when they undergo chemical reactions. This makes them more likely to react with non-metals and form compounds.
Conclusion
Based on the given information, the element Y with atomic number 12 is classified as a metal. It exhibits the characteristic properties of metals, such as metallic luster, conductivity, malleability, ductility, and reactivity.
Which of the following inequalities accurately describes the relationship between the two ionic species in terms of size?- a)Se2- > p5-
- b)LI+ > At-
- c)As3- > Y3+
- d)l- < CA2+
Correct answer is option 'C'. Can you explain this answer?
Which of the following inequalities accurately describes the relationship between the two ionic species in terms of size?
a)
Se2- > p5-
b)
LI+ > At-
c)
As3- > Y3+
d)
l- < CA2+
|
|
Ayesha Joshi answered |
In comparing ionic radii, there are two guiding rules. First, in comparing isoelectronic species, anions are always bigger than the cations such that A3- > B2- > C- > noble gas > X+ > Y2 + > Z3
Secondly, in comparing elements in a group, the ionic radii always increase going down the group or with each extra energy level such that l- > Br- > Cl- > F-
For l- and Ca2+ using the isoelectronic trend, we know that the iodide ion is larger than the barium cation. Since calcium and barium are in the same group, it can be deduced that calcium is smaller than barium. I⁻ must be larger than Ca2+
For At- and Li+ start superscript, plus, end superscript, using the isoelectronic trend, we know that the astatine ion is larger than the cesium cation. Since cesium and lithium are in the same group, it can be deduced that lithium is smaller than cesium. At- must be larger than Li+
For Se2- and p3-, since they are neither isoelectronic or in the same group, it cannot be stated that Se2- > p3-. In fact Se2- < p3- with values of 198 pm and 212 pm.
As for As3- and Y3+, they are both isoelectronic. Anions are larger than corresponding isoelectronic cations, so it can be said with certainty that has As3- a larger ionic radius than Y3+.
Which of the following statements does NOT accurately describe the atomic trends?- a)Moving up, electronegativity increases due to the shortening distance between the nucleus and valence electron shell.
- b)Moving down, effective nuclear charge decreases due to increasing electron shells.
- c)Moving across from left to right, ionic radius decreases, increases, then decreases due to the switch from cationic to anionic species.
- d)Moving across from left to right, second ionization energy increases due to increasing nuclear charge.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements does NOT accurately describe the atomic trends?
a)
Moving up, electronegativity increases due to the shortening distance between the nucleus and valence electron shell.
b)
Moving down, effective nuclear charge decreases due to increasing electron shells.
c)
Moving across from left to right, ionic radius decreases, increases, then decreases due to the switch from cationic to anionic species.
d)
Moving across from left to right, second ionization energy increases due to increasing nuclear charge.
|
|
Ayesha Joshi answered |
Effective nuclear charge increases moving across and decreases moving down.
Ionic radius decreases starting from the +1 to +3, sometimes +4, ion. Progressing to either the -4 or -3 ion, there is an increase in ionic radius since the anions are larger than the cations. Progressing from the -3 to the -1 ion, the radius decreases again.
Electronegativity increases moving across and decreases moving down.
The first ionization energy increases moving across and decreases moving down. For the second ionization energy, in period 2, lithium has the highest 2nd ionization energy because the electron is being removed from the noble gas configuration.
Which of the following statements describes a correct step in the formation of an ionic bond between sodium and chlorine?- a)Removing an electron from sodium (ionization energy) will provide energy for ionic bond formation.
- b)Adding an electron to chlorine (electron affinity) will require energy for ionic bond formation.
- c)The Coulombic potential between the ions (lattice energy) will release energy.
- d)Overcoming the Pauli repulsion from the overlap of wavefunctions of core electrons will release energy.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements describes a correct step in the formation of an ionic bond between sodium and chlorine?
a)
Removing an electron from sodium (ionization energy) will provide energy for ionic bond formation.
b)
Adding an electron to chlorine (electron affinity) will require energy for ionic bond formation.
c)
The Coulombic potential between the ions (lattice energy) will release energy.
d)
Overcoming the Pauli repulsion from the overlap of wavefunctions of core electrons will release energy.
|
|
Ayesha Joshi answered |
In order for the formation of an ionic bond to be favorable, the energy released must be greater than the energy consumed. Essentially, the cation and anion separately are formed and then brought together for bond formation.
Ionization energy is endothermic, so it would require energy. No energy is recovered from the ionization energy of sodium.
Electron affinity is exothermic, so it would release and provide energy for bond formation.
Once the ions are formed, they have to be brought together for bond formation. When the two charges are brought within picometers of each other, their electron shells which are both negatively charged will exert a certain repulsion on each other, and this repulsion will require energy to overcome.
However, the Pauli repulsion energy is small compared to the energy released in the loss in electrostatic or Coulombic potential when the opposite charges are brought together.
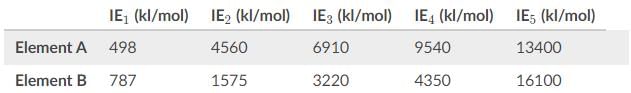
Based on the values for the first through fifth ionization energies in the table, what is the most likely identity of Element A and B?
- a)Ca and S
- b)Na and Si
- c)K and P
- d)Ne and Al
Correct answer is option 'B'. Can you explain this answer?
Based on the values for the first through fifth ionization energies in the table, what is the most likely identity of Element A and B?


a)
Ca and S
b)
Na and Si
c)
K and P
d)
Ne and Al

|
Orion Classes answered |
Ionization energies increase moving across a period, as a rule of thumb, since we are removing an electron first from a neutral atom then from a cation. To reiterate, second ionization energies are always larger than first ionization energies and are roughly double.
In analyzing the data for element A, look for the biggest gap. One way to approach this is to look at the factor between ionization energies.
The second ionization energy is almost 10 times as great as the first. As for the other ionization energies, the factor is only somewhere between 1 and 2.
The significance of the high amount of energy for IE2, is to due to the stability of the noble gas configuration. One electron was removed to reach IE2, so element A must be in group I.
As for element B, the doubling of IE1 to get IE2 is to be expected. The large gap does not occur until reaching IE5 whereby IE4 increases by a factor of almost 4.
Which of the following statements most accurately describes the characteristics of the transition metals?- a)The transition metals have little variability in their ionization energies and electronegativities due to their similar valence electron shells.
- b)Transition metals are characterized by the formation of many diamagnetic compounds due to the presence of unpaired d electrons.
- c)One difference between transition metals and main group metals is the latter’s ability to form coordination complexes.
- d)Transition metals are characterized by many oxidation states due to the high reactivity of the unpaired d electrons.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements most accurately describes the characteristics of the transition metals?
a)
The transition metals have little variability in their ionization energies and electronegativities due to their similar valence electron shells.
b)
Transition metals are characterized by the formation of many diamagnetic compounds due to the presence of unpaired d electrons.
c)
One difference between transition metals and main group metals is the latter’s ability to form coordination complexes.
d)
Transition metals are characterized by many oxidation states due to the high reactivity of the unpaired d electrons.
|
|
Ayesha Joshi answered |
Transition metals are the ones which form one or more stable ions which have incompletely filled d orbitals. The filling of the d orbitals imparts particular properties to the transition metals.
Transition metals are characterized by the formation of many paramagnetic compounds due to the presence of unpaired
d electrons. Diamagnetic compounds have paired electrons and do not possess any magnetic capabilities.
d electrons. Diamagnetic compounds have paired electrons and do not possess any magnetic capabilities.
Transition metals also are characterized by many oxidation states due to the low reactivity of the unpaired d electrons. High reactivity would limit the number of oxidation states.
One difference between transition metals and main group metals is that transition metals can form coordination complexes, while main group metals tend to form binary compounds like CaCl₂.
The transition metals have little variability in their ionization energies and electronegativities due to their similar valence electron shells, which is the s shell. Even though, for instance, 3d fills after 4s, when forming ions, we remove from the highest energy orbital or the s orbital.
Which of the following statements about atomic radii accurately describes the data below?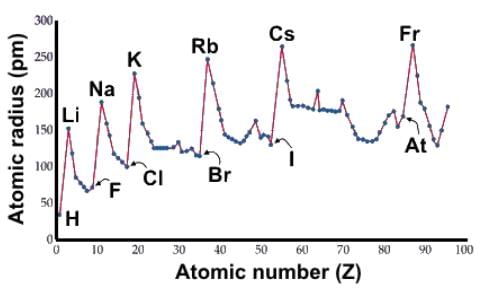
- a)The elements right after the d-block experience an expansion due to the repulsion created by the d electrons such that period 4 non metals are substantially larger than period 3 nonmetals.
- b)The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.
- c)The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.
- d)The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements about atomic radii accurately describes the data below?

a)
The elements right after the d-block experience an expansion due to the repulsion created by the d electrons such that period 4 non metals are substantially larger than period 3 nonmetals.
b)
The minima of the graph correspond to the halides because they have the greatest effective nuclear charge.
c)
The transition metals have relatively the same atomic size moving across the group because their valence shell, which is the s orbital, essentially remains the same.
d)
The peaks of the graph correspond to the noble gas configurations because they have fully-filled orbitals.

|
Orion Classes answered |
Solution:
- The atomic size of transition metals remains relatively consistent across a period.
- This consistency is because the valence shell primarily involves the s orbital, which does not change much.
- As a result, the atomic radii of transition metals do not significantly increase or decrease across the group.
Which of the following does NOT depend on the attraction of the bonding pair towards the nucleus?- a)The repulsion by the electrons in the same valence shell
- b)The amount of shielding by inner shell electrons
- c)The distance from the nucleus
- d)The number of protons in the nucleus
Correct answer is option 'A'. Can you explain this answer?
Which of the following does NOT depend on the attraction of the bonding pair towards the nucleus?
a)
The repulsion by the electrons in the same valence shell
b)
The amount of shielding by inner shell electrons
c)
The distance from the nucleus
d)
The number of protons in the nucleus

|
Orion Classes answered |
The attraction of the bonding pair towards the nucleus is influenced by several factors:
- Repulsion by electrons in the same valence shell: This is due to electron-electron repulsion and does not depend on attraction to the nucleus.
- The amount of shielding by inner shell electrons: This affects how much of the nuclear charge is felt by the bonding pair.
- The distance from the nucleus: Greater distance generally weakens the attraction.
- The number of protons in the nucleus: More protons increase the nuclear charge, enhancing attraction.
Thus, option A, repulsion by electrons in the same valence shell, does not depend on the attraction to the nucleus.
Chapter doubts & questions for Periodic Table (GC) - MCAT Chemical and Physical Foundations 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Periodic Table (GC) - MCAT Chemical and Physical Foundations in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
MCAT Chemical and Physical Foundations
336 videos|223 docs|109 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily









