All Exams >
MCAT >
MCAT Chemical and Physical Foundations >
All Questions
All questions of Rate Processes – Kinetics and Equilibrium (GC) for MCAT Exam
Ca(HCO3)2 is strongly heated and after equilibrium is attained, temperature changed to 25° C.
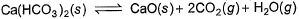
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is- a)36 atm
- b)18 atm
- c)12 atm
- d)6 atm
Correct answer is 'D'. Can you explain this answer?
Ca(HCO3)2 is strongly heated and after equilibrium is attained, temperature changed to 25° C.
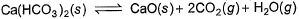
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is
a)
36 atm
b)
18 atm
c)
12 atm
d)
6 atm
|
|
Mira Joshi answered |
The reaction is as follow:-
Ca(HCO3)2(s)⇌CaO(s) + 2CO2 (g) + H2O(g)
At 25° C H2O goes in liquid state
Kp = (PCaO)1×(PCO2)2
(PCa(HCO3)2)
Since, Ca(HCO3)2, CaO and H2O are not in gaseous state, so their partial pressure is taken 1.
Putting all values, we have
36 = (PCO2)2
Or PCO2 = 6 atm
Ca(HCO3)2(s)⇌CaO(s) + 2CO2 (g) + H2O(g)
At 25° C H2O goes in liquid state
Kp = (PCaO)1×(PCO2)2
(PCa(HCO3)2)
Since, Ca(HCO3)2, CaO and H2O are not in gaseous state, so their partial pressure is taken 1.
Putting all values, we have
36 = (PCO2)2
Or PCO2 = 6 atm
Following equilibrium is set up at 298 K in a 1 L flask.
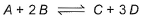
If one starts with 2 moles of A and 1 mole of B, it is found that moles of B and D are equal.Thus Kc is - a)9.0
- b)15.0
- c)3.0
- d)0.0667
Correct answer is option 'B'. Can you explain this answer?
Following equilibrium is set up at 298 K in a 1 L flask.
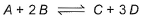
If one starts with 2 moles of A and 1 mole of B, it is found that moles of B and D are equal.Thus Kc is
If one starts with 2 moles of A and 1 mole of B, it is found that moles of B and D are equal.Thus Kc is
a)
9.0
b)
15.0
c)
3.0
d)
0.0667

|
Sushil Kumar answered |
For the equilibrium reaction:
A+2B ⇌ 2C+D
volume of flask = 1L
Initial moles of A = 2 mol
initial concentration of A=[A]i = 2 M
initial mole of B = 1 mol
[B]i = 1 M
[A]eq = 2-x, [B]eq = 1-2x, [C]eq = x, [D]eq = 3x
Given [D]eq = 1 * 1L
= 1 M
Thus x = 1M
[A]eq = 1, [B]eq = -1, [C]eq = 1, [D] = 3
Kc = {([D]eq)3 * ([C]eq)}/{[A]eq * ([B]eq)2}
= Kc = {(3)3*1}/{1*(-1)2}
= 27/1
= 27
A+2B ⇌ 2C+D
volume of flask = 1L
Initial moles of A = 2 mol
initial concentration of A=[A]i = 2 M
initial mole of B = 1 mol
[B]i = 1 M
[A]eq = 2-x, [B]eq = 1-2x, [C]eq = x, [D]eq = 3x
Given [D]eq = 1 * 1L
= 1 M
Thus x = 1M
[A]eq = 1, [B]eq = -1, [C]eq = 1, [D] = 3
Kc = {([D]eq)3 * ([C]eq)}/{[A]eq * ([B]eq)2}
= Kc = {(3)3*1}/{1*(-1)2}
= 27/1
= 27
Graphite is added to a vessel that contains CO2(g) at a pressure of 0.830 atm at a certain high temperature. The pressure rises due to a reaction that produces CO (g). The total pressure reaches an equilibrium value of 1.366 atm. Calculate the equilibrium constant of the following reaction.
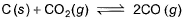
- a)2.909 atm
- b)6.909 atm
- c)4.909 atm
- d)3.909 atm
Correct answer is option 'D'. Can you explain this answer?
Graphite is added to a vessel that contains CO2(g) at a pressure of 0.830 atm at a certain high temperature. The pressure rises due to a reaction that produces CO (g). The total pressure reaches an equilibrium value of 1.366 atm. Calculate the equilibrium constant of the following reaction.
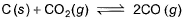
a)
2.909 atm
b)
6.909 atm
c)
4.909 atm
d)
3.909 atm
|
|
Hansa Sharma answered |

Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Vapour pressure of NH4HS (s)is 20 mm at 25°C, for
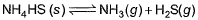
Total pressure when NH4HS (s) dissociates at 25°Cin a vessel which already contains H2S (g)at a pressure of 15 mm, is- a)25 mm
- b)50 mm
- c)5 mm
- d)10 mm
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Vapour pressure of NH4HS (s)is 20 mm at 25°C, for
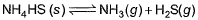
Total pressure when NH4HS (s) dissociates at 25°Cin a vessel which already contains H2S (g)at a pressure of 15 mm, is
Total pressure when NH4HS (s) dissociates at 25°Cin a vessel which already contains H2S (g)at a pressure of 15 mm, is
a)
25 mm
b)
50 mm
c)
5 mm
d)
10 mm
|
|
Hansa Sharma answered |
The correct answer is Option A.
NH4HS -------> NH3 + H2S
Let P be the pressure at eq. of NH3 and H2S.
Therefore, Kp = P2
= (20 / 2)2
= 100 mm
= 100
Also, Kp = (15 + P) (P)
100 = 15 P + P2
P2 + 15 P – 100 = 0
P = 5
Total pressure = 15 + 2(P)
= 15 + 2(5)
= 25 mm
NH4HS -------> NH3 + H2S
Let P be the pressure at eq. of NH3 and H2S.
Therefore, Kp = P2
= (20 / 2)2
= 100 mm
= 100
Also, Kp = (15 + P) (P)
100 = 15 P + P2
P2 + 15 P – 100 = 0
P = 5
Total pressure = 15 + 2(P)
= 15 + 2(5)
= 25 mm
In a reaction, A + B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (Aand B) are doubled, rate law for the reaction can be written as - a)Rate = k[A] [B]
- b)Rate = k[A]2[B]
- c)Rate = k[A][B]2
- d)Rate = k[A]2[B]2
Correct answer is option 'B'. Can you explain this answer?
In a reaction, A + B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (Aand B) are doubled, rate law for the reaction can be written as
a)
Rate = k[A] [B]
b)
Rate = k[A]2[B]
c)
Rate = k[A][B]2
d)
Rate = k[A]2[B]2

|
Dr Manju Sen answered |
The correct answer is option B
When conc. of B is doubled, the rate is doubled.
So, Order w.r.t. [B] is 1.
The rate increases by a factor of 8 when conc. of [A] and [B] are doubled.
So, Order w.r.t. [A] is 2.
Overall Rate is
Rate=k[A]2[B]
When conc. of B is doubled, the rate is doubled.
So, Order w.r.t. [B] is 1.
The rate increases by a factor of 8 when conc. of [A] and [B] are doubled.
So, Order w.r.t. [A] is 2.
Overall Rate is
Rate=k[A]2[B]
Following equilibrium is set up at 1000 K and 1 bar in a 5 L flask,
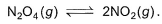
At equilibrium, NO2 is 50% o f the total volume. Thus, equilibrium constant Kc is - a)0.133
- b)0.266
- c)0.200
- d)0.400
Correct answer is option 'A'. Can you explain this answer?
Following equilibrium is set up at 1000 K and 1 bar in a 5 L flask,
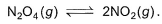
At equilibrium, NO2 is 50% o f the total volume. Thus, equilibrium constant Kc is
At equilibrium, NO2 is 50% o f the total volume. Thus, equilibrium constant Kc is
a)
0.133
b)
0.266
c)
0.200
d)
0.400

|
Manish Aggarwal answered |
The correct answer is Option A.
N2O4 ⇌ 2NO2
Initial 1 0
Equilibrium 1−x 2x
N2O4 ⇌ 2NO2
Initial 1 0
Equilibrium 1−x 2x
Total moles = 1 - x + 2x
NO2 is 50% of the total volume when equilibrium is set up.
Thus, the volume fraction (at equilibrium) of NO2 = 50/100 = 0.5 = ½
So, 2x / (1+x) = ½
=> x = ⅓
Thus, the volume fraction (at equilibrium) of NO2 = 50/100 = 0.5 = ½
So, 2x / (1+x) = ½
=> x = ⅓
For 1 litre;
Kc = [NO2] / [N2O4]
= [4*(1/9)] / [⅔]
= 0.66;
Kc = [NO2] / [N2O4]
= [4*(1/9)] / [⅔]
= 0.66;
For 5 litres;
Kc = 0.66 / 5
= 0.133
Thus, option A is correct.
Kc = 0.66 / 5
= 0.133
Thus, option A is correct.
Assume following equilibria when total pressure set up in each are equal to 1 atm, and equilibrium constant (Kp) as K1; K2 and K3
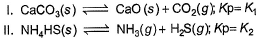
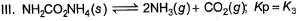
Thus,- a) K1 = K2 = K3
- b)K1 < K2 < K3
- c)K3 < K2 < K1
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Assume following equilibria when total pressure set up in each are equal to 1 atm, and equilibrium constant (Kp) as K1; K2 and K3
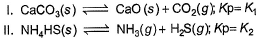
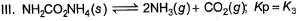
Thus,
Thus,
a)
K1 = K2 = K3
b)
K1 < K2 < K3
c)
K3 < K2 < K1
d)
None of these

|
Pioneer Academy answered |
The correct answer is option C
CaCO3 → CaO + CO2
Kp = k1 = Pco2
total pressure of container P
k1 = p
NH4HS → NH3 + H2S
PNH3 = PH2S = P0
P0 + P0 = p (total pressure)
P0 = p/2
k2 = kp = [PNH3][PH2s] p24
NH2CoNH2 → 2NH3 + CO2
PNH3 = 2P0 PCO2 = P0
2P0 + P0 = P
CaCO3 → CaO + CO2
Kp = k1 = Pco2
total pressure of container P
k1 = p
NH4HS → NH3 + H2S
PNH3 = PH2S = P0
P0 + P0 = p (total pressure)
P0 = p/2
k2 = kp = [PNH3][PH2s] p24
NH2CoNH2 → 2NH3 + CO2
PNH3 = 2P0 PCO2 = P0
2P0 + P0 = P
Ca(HCO3)2 decomposes as,
Ca (HCO3)2(s) ⇌ CaCO3(s) + H2O(g) + CO2(g)
Equilibrium pressure is found to be 0.12 bar. What is pco2 if the reaction mixture also contains H2O(g)at 0.20 bar?
- a) 0.20 bar
- b) 0.017 ba
- c)0.040 bar
- d)0.10 bar
Correct answer is option 'B'. Can you explain this answer?
Ca(HCO3)2 decomposes as,
Ca (HCO3)2(s) ⇌ CaCO3(s) + H2O(g) + CO2(g)
Equilibrium pressure is found to be 0.12 bar. What is pco2 if the reaction mixture also contains H2O(g)at 0.20 bar?
Equilibrium pressure is found to be 0.12 bar. What is pco2 if the reaction mixture also contains H2O(g)at 0.20 bar?
a)
0.20 bar
b)
0.017 ba
c)
0.040 bar
d)
0.10 bar

|
Top Rankers answered |

At 1000 K, pressure of CO2 in equilibrium with CaCO3 and CaO is equal to 2.105 atm. The equilibrium constant for the reaction,
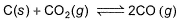
is 1.9 at the same temperature when pressure are in atm. Solid C, CaO, and CaCO3 are mixed and allowed to come to equilibrium at 1000 K in a closed vessel.Q. What is the pressure of CO (g)at equilibrium (in atm)?
Correct answer is '2'. Can you explain this answer?
At 1000 K, pressure of CO2 in equilibrium with CaCO3 and CaO is equal to 2.105 atm. The equilibrium constant for the reaction,
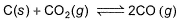
is 1.9 at the same temperature when pressure are in atm. Solid C, CaO, and CaCO3 are mixed and allowed to come to equilibrium at 1000 K in a closed vessel.
is 1.9 at the same temperature when pressure are in atm. Solid C, CaO, and CaCO3 are mixed and allowed to come to equilibrium at 1000 K in a closed vessel.
Q. What is the pressure of CO (g)at equilibrium (in atm)?

|
Learners Habitat answered |
K= (partial pressure of co2/(partial pressure of co2)
since k =1.9
So 1.9 = (partial pressure of co)2/2.105
(partial pressure of co)2 =2.105×1.9
= 3.99 = 4
(partial pressure of co) =2
since k =1.9
So 1.9 = (partial pressure of co)2/2.105
(partial pressure of co)2 =2.105×1.9
= 3.99 = 4
(partial pressure of co) =2
The equilibrium constant for the following reaction, is 1.6 x 105 at 1024 K.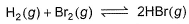
HBr (g)at 10.0 bar is introduced into a sealed container at 1024 K. Thus, partial pressure of H2(g)and Br2(g), together is- a)10 bar
- b)0.05 bar
- c)0.025 bar
- d)0.10 bar
Correct answer is option 'A'. Can you explain this answer?
The equilibrium constant for the following reaction, is 1.6 x 105 at 1024 K.
HBr (g)at 10.0 bar is introduced into a sealed container at 1024 K. Thus, partial pressure of H2(g)and Br2(g), together is
a)
10 bar
b)
0.05 bar
c)
0.025 bar
d)
0.10 bar
|
|
Rahul Bansal answered |
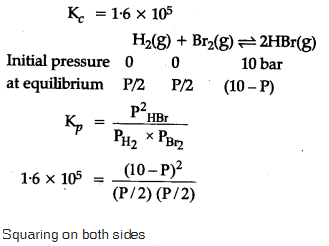
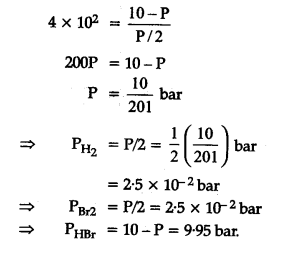
=> 10 bar approximately
A sample of N2O4(g)with a pressure of 1.00 atm is placed in a flask. When equilibrium is reached, 20% of N2O4(g)has been converted to NO2(g)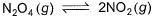 If the original pressure is made 10% of the earlier pressure, then per cent dissociation will be
If the original pressure is made 10% of the earlier pressure, then per cent dissociation will be- a)20%
- b)42%
- c)54%
- d)62%
Correct answer is option 'C'. Can you explain this answer?
A sample of N2O4(g)with a pressure of 1.00 atm is placed in a flask. When equilibrium is reached, 20% of N2O4(g)has been converted to NO2(g)
If the original pressure is made 10% of the earlier pressure, then per cent dissociation will be
a)
20%
b)
42%
c)
54%
d)
62%

|
Lohit Matani answered |
Correct answer is A.

For the reversible reaction, 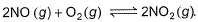
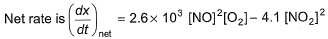
In a reaction vessel, [NO]= [O2]= 0.01 mol L-1 and [NO2]= 0.1 mol L-1 then above reaction is - a)shifted in forward direction
- b)shifted in backward direction
- c)in equilibrium
- d)not predictable in the absence of required data
Correct answer is option 'A'. Can you explain this answer?
For the reversible reaction, 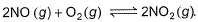
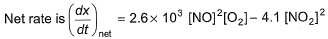
In a reaction vessel, [NO]= [O2]= 0.01 mol L-1 and [NO2]= 0.1 mol L-1 then above reaction is
In a reaction vessel, [NO]= [O2]= 0.01 mol L-1 and [NO2]= 0.1 mol L-1 then above reaction is
a)
shifted in forward direction
b)
shifted in backward direction
c)
in equilibrium
d)
not predictable in the absence of required data
|
|
Lavanya Menon answered |
On substituting the values of conc. of NO, O2 and NO2 in given rate equation, we get a +ve (positive) value indicating that the reaction takes place in forward direction.
If 75% of a first order reaction was completed in 32 min, then 50% of the reaction was completed in- a)24 min
- b)4 min
- c)16 min
- d)8 min
Correct answer is option 'C'. Can you explain this answer?
If 75% of a first order reaction was completed in 32 min, then 50% of the reaction was completed in
a)
24 min
b)
4 min
c)
16 min
d)
8 min

|
Avi Chawla answered |
75% completion means 2 half lifes so 50% completion means only one half life.
Which of the following is an example of a zero-order reaction?- a)Radioactive decay
- b)First-order decay
- c)Second-order reaction
- d)Photochemical reaction
Correct answer is option 'A'. Can you explain this answer?
Which of the following is an example of a zero-order reaction?
a)
Radioactive decay
b)
First-order decay
c)
Second-order reaction
d)
Photochemical reaction
|
|
Harper White answered |
Zero-Order Reaction
A zero-order reaction is a type of chemical reaction in which the rate of the reaction is independent of the concentrations of the reactants. This means that the reaction proceeds at a constant rate, regardless of how much reactant is present. The rate equation for a zero-order reaction is given by:
Rate = k
where k is the rate constant.
Radioactive Decay
Radioactive decay is an example of a zero-order reaction. It is the process by which unstable atomic nuclei spontaneously break apart, releasing radiation and transforming into a different element. The rate of radioactive decay is determined solely by the decay constant, which is a characteristic property of the radioactive material.
Explanation
In a zero-order reaction, the rate of the reaction does not depend on the concentration of the reactants. This means that even if the concentration of the reactant is doubled or halved, the rate of the reaction will remain the same.
Radioactive decay follows this behavior because it is a first-order reaction with respect to the radioactive material. The rate of decay is determined solely by the decay constant, which is a property of the specific radioactive isotope. The decay constant represents the probability of a radioactive atom decaying per unit time and is independent of the concentration of the radioactive material.
For example, if we have a sample of radioactive material with an initial concentration of 1 mol/L, the rate of decay will be a constant value determined by the decay constant. If we double the concentration of the radioactive material to 2 mol/L, the rate of decay will still be the same constant value. Similarly, if we halve the concentration to 0.5 mol/L, the rate of decay will remain unchanged.
Conclusion
Radioactive decay is an example of a zero-order reaction because the rate of decay is independent of the concentration of the radioactive material. The rate of decay is determined solely by the decay constant, which is a characteristic property of the radioactive isotope.
A zero-order reaction is a type of chemical reaction in which the rate of the reaction is independent of the concentrations of the reactants. This means that the reaction proceeds at a constant rate, regardless of how much reactant is present. The rate equation for a zero-order reaction is given by:
Rate = k
where k is the rate constant.
Radioactive Decay
Radioactive decay is an example of a zero-order reaction. It is the process by which unstable atomic nuclei spontaneously break apart, releasing radiation and transforming into a different element. The rate of radioactive decay is determined solely by the decay constant, which is a characteristic property of the radioactive material.
Explanation
In a zero-order reaction, the rate of the reaction does not depend on the concentration of the reactants. This means that even if the concentration of the reactant is doubled or halved, the rate of the reaction will remain the same.
Radioactive decay follows this behavior because it is a first-order reaction with respect to the radioactive material. The rate of decay is determined solely by the decay constant, which is a property of the specific radioactive isotope. The decay constant represents the probability of a radioactive atom decaying per unit time and is independent of the concentration of the radioactive material.
For example, if we have a sample of radioactive material with an initial concentration of 1 mol/L, the rate of decay will be a constant value determined by the decay constant. If we double the concentration of the radioactive material to 2 mol/L, the rate of decay will still be the same constant value. Similarly, if we halve the concentration to 0.5 mol/L, the rate of decay will remain unchanged.
Conclusion
Radioactive decay is an example of a zero-order reaction because the rate of decay is independent of the concentration of the radioactive material. The rate of decay is determined solely by the decay constant, which is a characteristic property of the radioactive isotope.
Kc forthe decomposition of NH4HS(s) is 1.8x 10-4 at 25°C.
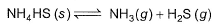
If the system already contains [NH3] = 0.020 M, then when equilibrium is reached, molar concentration are
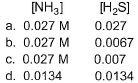
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
Kc forthe decomposition of NH4HS(s) is 1.8x 10-4 at 25°C.
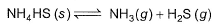
If the system already contains [NH3] = 0.020 M, then when equilibrium is reached, molar concentration are

If the system already contains [NH3] = 0.020 M, then when equilibrium is reached, molar concentration are
a)
a
b)
b
c)
c
d)
d

|
Sushil Kumar answered |
NH4HS (s) ⇋ NH3 (g) + H2S (g)
Initial 1 - -
At eqm 1-x x+0.02 x
Kc = [NH3][H2S] (Since NH4HS is solid, we ignore it.)
1.8×10-4 = (x+0.02)(x)
x2+0.02x-1.8×10-4 = 0
Applying quadratic formula; x = -0.02+√{(0.02)2-4×1.8×10-4}
= 0.033-0.020/2 = 0.0065
Therefore, concn of NH3 at equilibrium = x+0.020 = 0.0265
concn of H2S at equilibrium = x = 0.0065
So, option b is correct
Initial 1 - -
At eqm 1-x x+0.02 x
Kc = [NH3][H2S] (Since NH4HS is solid, we ignore it.)
1.8×10-4 = (x+0.02)(x)
x2+0.02x-1.8×10-4 = 0
Applying quadratic formula; x = -0.02+√{(0.02)2-4×1.8×10-4}
= 0.033-0.020/2 = 0.0065
Therefore, concn of NH3 at equilibrium = x+0.020 = 0.0265
concn of H2S at equilibrium = x = 0.0065
So, option b is correct
Equilibrium constant for the reaction PCL5⇋PCL3+CL2 is 0.0205 at 230°C and 1 atmospheric pressure if at equilibrium concentration of PCL5 is 0.235 moles liter−1liter-1and that of CL2= 0.028 moleslit−1lit-1 then conc. of PCL3 at equilibrium is- a)0.0174
- b)0.174
- c)0.0348
- d)1.74
Correct answer is option 'A'. Can you explain this answer?
Equilibrium constant for the reaction PCL5⇋PCL3+CL2 is 0.0205 at 230°C and 1 atmospheric pressure if at equilibrium concentration of PCL5 is 0.235 moles liter−1liter-1and that of CL2= 0.028 moleslit−1lit-1 then conc. of PCL3 at equilibrium is
a)
0.0174
b)
0.174
c)
0.0348
d)
1.74
|
|
Mira Sharma answered |

The rate is independent of the concentration of the reactants in- a)First order
- b)Second order
- c)Third order
- d)Zero order
Correct answer is option 'D'. Can you explain this answer?
The rate is independent of the concentration of the reactants in
a)
First order
b)
Second order
c)
Third order
d)
Zero order
|
|
Naiham Difoesa answered |
Bcz rate is proportional to the zeroth power of the concentration of reactant.
The unit of rate constant for a first order reaction is- a)Mol/L
- b)Mol2 / L2 / S2
- c)S-1
- d)Mol/L/S
Correct answer is option 'C'. Can you explain this answer?
The unit of rate constant for a first order reaction is
a)
Mol/L
b)
Mol2 / L2 / S2
c)
S-1
d)
Mol/L/S
|
|
Nikita Singh answered |
The correct answer is Option C.
Let R be the rate of reaction.
For first order reaction,
R=K[A]1
⇒K=R[A]-1
Whereas, K and [A] are rate constant and initial concentration of reactant respectively.
Therefore,
Unit of rate constant =(mol L-1)1-nsec-1
For first order reaction, n=1
Unit of rate constant = sec-1
Hence the unit of rate constant for first order reaction is sec-1.
For first order reaction,
R=K[A]1
⇒K=R[A]-1
Whereas, K and [A] are rate constant and initial concentration of reactant respectively.
Therefore,
Unit of rate constant =(mol L-1)1-nsec-1
For first order reaction, n=1
Unit of rate constant = sec-1
Hence the unit of rate constant for first order reaction is sec-1.
Passage ISolid ammonium chloride is in equilibrium with ammonia and hydrogen chloride gases
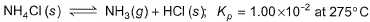
0.980 g of solid NH4CI is taken in a closed vessel of 1 L capacity and heated to 275° C.Q. Percentage decomposition of the original sample is- a)24.81%
- b)6.24%
- c)3.12%
- d)12.13%
Correct answer is option 'D'. Can you explain this answer?
Passage I
Solid ammonium chloride is in equilibrium with ammonia and hydrogen chloride gases
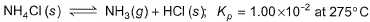
0.980 g of solid NH4CI is taken in a closed vessel of 1 L capacity and heated to 275° C.
0.980 g of solid NH4CI is taken in a closed vessel of 1 L capacity and heated to 275° C.
Q. Percentage decomposition of the original sample is
a)
24.81%
b)
6.24%
c)
3.12%
d)
12.13%

|
Knowledge Hub answered |
The state of HCl is given wrong. It will be in gaseous state.
So, the reaction be like;-
NH4Cl(s) ⇌ NH3(g) + HCl(g) kp = 1.00×10-2 at 275° C
Kp = kc(RT)2
1.00×10-2 = kc(0.0821×548)2
Or kc = 4.94×10-6
NH4Cl(s) ⇌ NH3(g) + HCl(g)
Initial 1 - -
At eqm 1-x x x
Kc = x2
x = √(4.94×10-6)
= 2.22×10-3
Therefore, NH4Cl dissociated at eqm = 2.22×10-3 × 53.5 = 0.118
%age decomposition = 0.118/0.980×100 = 12.13%
So, the reaction be like;-
NH4Cl(s) ⇌ NH3(g) + HCl(g) kp = 1.00×10-2 at 275° C
Kp = kc(RT)2
1.00×10-2 = kc(0.0821×548)2
Or kc = 4.94×10-6
NH4Cl(s) ⇌ NH3(g) + HCl(g)
Initial 1 - -
At eqm 1-x x x
Kc = x2
x = √(4.94×10-6)
= 2.22×10-3
Therefore, NH4Cl dissociated at eqm = 2.22×10-3 × 53.5 = 0.118
%age decomposition = 0.118/0.980×100 = 12.13%
Study of rate of chemical reaction is known as- a)Chemical thermodynamics
- b)Chemical kinetics
- c)Chemical equilibrium
- d)Chemical change
Correct answer is option 'B'. Can you explain this answer?
Study of rate of chemical reaction is known as
a)
Chemical thermodynamics
b)
Chemical kinetics
c)
Chemical equilibrium
d)
Chemical change

|
Sarthak Khanna answered |
Chemical Kinetics deals with rate of a Chemical reactions.
For the equilibrium,
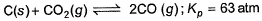
at 1000 K. If at equilibrium pCO = 10 then total pressure at equilibrium is
then total pressure at equilibrium is - a)6.30 atm
- b)0.63 atm
- c)6.93 atm
- d)69.3 atm
Correct answer is option 'C'. Can you explain this answer?
For the equilibrium,
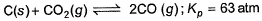
at 1000 K. If at equilibrium pCO = 10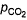 then total pressure at equilibrium is
then total pressure at equilibrium is
at 1000 K. If at equilibrium pCO = 10
a)
6.30 atm
b)
0.63 atm
c)
6.93 atm
d)
69.3 atm
|
|
Lavanya Menon answered |
C(s) + CO2(g) <=========> 2CO(g)
Kp = pCO2/pCO2
GIven Kp = 63 and pCO = 10pCO2
Putting the value of pCO in above equation,
63 = 100(pCO2)2/pCO2
Or pCO2 = 0.63
pCO = 6.3
Therefore, total pressure = 6.3+0.63 = 6.93 atm
Kp = pCO2/pCO2
GIven Kp = 63 and pCO = 10pCO2
Putting the value of pCO in above equation,
63 = 100(pCO2)2/pCO2
Or pCO2 = 0.63
pCO = 6.3
Therefore, total pressure = 6.3+0.63 = 6.93 atm
Direction (Q. Nos. 21) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)Q. For the equilibrium in gaseous phase in 2 L flask we start with 2 moles of SO2 and 1 mole of O2 at 3 atm, 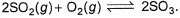
When equilibrium is attained, pressure changes to 2.5 atm. Hence, equilibrium constant Kc is
Correct answer is '4'. Can you explain this answer?
Direction (Q. Nos. 21) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q. For the equilibrium in gaseous phase in 2 L flask we start with 2 moles of SO2 and 1 mole of O2 at 3 atm, 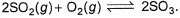
When equilibrium is attained, pressure changes to 2.5 atm. Hence, equilibrium constant Kc is
When equilibrium is attained, pressure changes to 2.5 atm. Hence, equilibrium constant Kc is
|
|
Om Desai answered |
The correct answer is 4
2SO2(g) + O2(g) ⇋ 2SO3
Initial moles 2 1
At equilibrium 2 - 2x 1 - x 2x
Net moles at equilibrium = 2 - 2x + 1 - x + 2x
=(3 - x)moles
Initial:
moles = 3,
Pressure = 3 atm,
Volume = 2L,
PV = nRT
3 x 2 = 3RT -------- 1
At equilibrium
Moles = 3 - x,
Pressure = 2.5 atm
Volume = 2L
P‘V = n’RT ---------- 2
Divide eqn 2 by 1
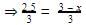
⇒2.5 = 3 - x
⇒x = 0.5
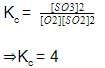
2SO2(g) + O2(g) ⇋ 2SO3
Initial moles 2 1
At equilibrium 2 - 2x 1 - x 2x
Net moles at equilibrium = 2 - 2x + 1 - x + 2x
=(3 - x)moles
Initial:
moles = 3,
Pressure = 3 atm,
Volume = 2L,
PV = nRT
3 x 2 = 3RT -------- 1
At equilibrium
Moles = 3 - x,
Pressure = 2.5 atm
Volume = 2L
P‘V = n’RT ---------- 2
Divide eqn 2 by 1
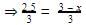
⇒2.5 = 3 - x
⇒x = 0.5
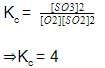
If a reaction proceeds with a uniform rate throughout, the reaction is- a)Third order
- b)Second order
- c)First order
- d)Zero order
Correct answer is option 'D'. Can you explain this answer?
If a reaction proceeds with a uniform rate throughout, the reaction is
a)
Third order
b)
Second order
c)
First order
d)
Zero order

|
Roshni Chavan answered |
Reaction Rate and Order:
The rate of a chemical reaction refers to how quickly reactants are consumed and products are formed. It is determined by the rate equation, which shows how the concentration of reactants influences the rate of the reaction. The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate equation.
Uniform Rate throughout the Reaction:
When a reaction proceeds with a uniform rate throughout, it means that the rate of the reaction remains constant over time. This implies that the concentration of the reactants does not affect the reaction rate. In other words, the reaction is not dependent on the concentration of the reactants.
Zero Order Reaction:
When the reaction rate is independent of the concentration of the reactants, it is referred to as a zero order reaction. In this case, the rate equation is expressed as:
Rate = k
where k is the rate constant. The rate of the reaction is solely determined by the value of the rate constant, and it does not change with changes in reactant concentrations.
Explanation:
In the given question, if the reaction proceeds with a uniform rate throughout, it indicates that the rate of the reaction does not depend on the concentration of the reactants. This is a characteristic of a zero order reaction.
The rate equation for a zero order reaction is Rate = k, where k is the rate constant. It means that the reaction proceeds at a constant rate, regardless of the initial concentrations of the reactants. The rate constant determines the speed of the reaction, and it remains constant throughout the reaction.
Therefore, the correct answer is option 'D' - Zero order.
The rate of a chemical reaction refers to how quickly reactants are consumed and products are formed. It is determined by the rate equation, which shows how the concentration of reactants influences the rate of the reaction. The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate equation.
Uniform Rate throughout the Reaction:
When a reaction proceeds with a uniform rate throughout, it means that the rate of the reaction remains constant over time. This implies that the concentration of the reactants does not affect the reaction rate. In other words, the reaction is not dependent on the concentration of the reactants.
Zero Order Reaction:
When the reaction rate is independent of the concentration of the reactants, it is referred to as a zero order reaction. In this case, the rate equation is expressed as:
Rate = k
where k is the rate constant. The rate of the reaction is solely determined by the value of the rate constant, and it does not change with changes in reactant concentrations.
Explanation:
In the given question, if the reaction proceeds with a uniform rate throughout, it indicates that the rate of the reaction does not depend on the concentration of the reactants. This is a characteristic of a zero order reaction.
The rate equation for a zero order reaction is Rate = k, where k is the rate constant. It means that the reaction proceeds at a constant rate, regardless of the initial concentrations of the reactants. The rate constant determines the speed of the reaction, and it remains constant throughout the reaction.
Therefore, the correct answer is option 'D' - Zero order.
Which among the following is an example of first order reaction?- a)Acid catalysed hydrolysis of ethyl acetate
- b)Formation and dissociation of ozone
- c)Inversion of cane sugar
- d)Decomposition of nitrogen pentoxide
Correct answer is option 'D'. Can you explain this answer?
Which among the following is an example of first order reaction?
a)
Acid catalysed hydrolysis of ethyl acetate
b)
Formation and dissociation of ozone
c)
Inversion of cane sugar
d)
Decomposition of nitrogen pentoxide

|
Pooja Pillai answered |
Decomposition of N2O5 is 1st order reaction.
Decomposition of H2O2 was studied by titration against KMnO4 solution. It was found that 0.4 mol of H2O2 was reduced to 0.2 mol in 20 min and to 0.1 mol in 40 min and to 0.05 mol after 1 hr, the order of reaction must be- a)3
- b)1
- c)0
- d)2
Correct answer is option 'B'. Can you explain this answer?
Decomposition of H2O2 was studied by titration against KMnO4 solution. It was found that 0.4 mol of H2O2 was reduced to 0.2 mol in 20 min and to 0.1 mol in 40 min and to 0.05 mol after 1 hr, the order of reaction must be
a)
3
b)
1
c)
0
d)
2

|
Om Kumar answered |
Decomposition of nH2O2 is 1st order.
Which among the following statement is not true for rate constant of a reaction?- a)Rate constant depend upon the concentration of the reactants
- b)Unit of rate constant depend upon the order of reaction
- c)Rate constant has a definite value at a particular temperature
- d)Rate constant changes with temperature
Correct answer is option 'A'. Can you explain this answer?
Which among the following statement is not true for rate constant of a reaction?
a)
Rate constant depend upon the concentration of the reactants
b)
Unit of rate constant depend upon the order of reaction
c)
Rate constant has a definite value at a particular temperature
d)
Rate constant changes with temperature

|
Anjana Sen answered |
Rate constant is independent of concentration of reactant.
Molecularity of a reaction
- a)Cannot be less than 2
- b)Can be zero
- c)Is always a natural number
- d)Can have a fractional values
Correct answer is option 'C'. Can you explain this answer?
Molecularity of a reaction
a)
Cannot be less than 2
b)
Can be zero
c)
Is always a natural number
d)
Can have a fractional values

|
Nandini Nair answered |
Molecularity is defined as the number of molecules, atoms, or radicals that must collide simultaneously in order for the reaction to take place. It is always a natural number and cannot be negative.
At 700 K and 350 bar, a 1 : 3 mixture of N2(g) and H2(g) reacts to form an equilibrium mixture containing X (NH3)= 0.50. Assuming ideal behaviour Kp for the equilibrium reaction, 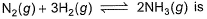
- a)2.03x 10-4
- b)3.55x 10-3
- c)1.02 x 10-4
- d)3.1 x 10-4
Correct answer is option 'D'. Can you explain this answer?
At 700 K and 350 bar, a 1 : 3 mixture of N2(g) and H2(g) reacts to form an equilibrium mixture containing X (NH3)= 0.50. Assuming ideal behaviour Kp for the equilibrium reaction, 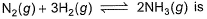
a)
2.03x 10-4
b)
3.55x 10-3
c)
1.02 x 10-4
d)
3.1 x 10-4

|
Knowledge Hub answered |
The correct answer is option A
2.03x 10-4
The given equation is :-
N2(g)+3H2(g) ⇌ 2NH3(g)
Initial moles : 1 3 0
At eqm ; (1−x) (3−3x) (2x)
(let)
Total moles of equation
=1 − x + 3 − 3x + 2x = (4−2x)
Now, X(NH3) =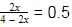
⇒ 2x = 2 − x
⇒ 3x = 2 ⇒ x = 0.66 =
32
Now, at equation, moles of N2= 1/3, moles of NH3 = 4/3
moles of H2 =3 − 2 = 1
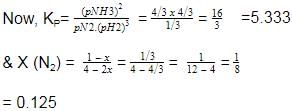
2.03x 10-4
The given equation is :-
N2(g)+3H2(g) ⇌ 2NH3(g)
Initial moles : 1 3 0
At eqm ; (1−x) (3−3x) (2x)
(let)
Total moles of equation
=1 − x + 3 − 3x + 2x = (4−2x)
Now, X(NH3) =
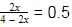
⇒ 2x = 2 − x
⇒ 3x = 2 ⇒ x = 0.66 =
32
Now, at equation, moles of N2= 1/3, moles of NH3 = 4/3
moles of H2 =3 − 2 = 1
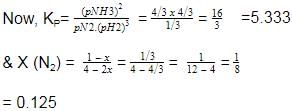
A first order reaction is 50% completed in 1.26 × 1014 s. How much time would it take for 100% completion?- a)1.26 × 1015 s
- b)2.52 × 1014 s
- c)2.52 × 1028 s
- d) infinite
Correct answer is option 'D'. Can you explain this answer?
A first order reaction is 50% completed in 1.26 × 1014 s. How much time would it take for 100% completion?
a)
1.26 × 1015 s
b)
2.52 × 1014 s
c)
2.52 × 1028 s
d)
infinite
|
|
Neha Chauhan answered |
The time taken for half the reaction to complete, i.e., the time in which the concentration of a reactant is reduced to half of its original value is called half-life period of the reaction. But it is impossible to perform 100% of the reaction. Whole of the substance never reacts because in every half-life, 50% of the substance reacts. Hence, time taken for 100% completion of a reaction is infinite.
At 273 K and 1 atm, 1 L of N2O4 (g) decomposes to NO2(g)a s given,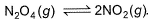 At equilibrium, original volume is 25% less than the existing volume. Percentage decomposition of N2O4 (g) is thus,
At equilibrium, original volume is 25% less than the existing volume. Percentage decomposition of N2O4 (g) is thus, - a)25%
- b)50%
- c)66.66%
- d)33.33%
Correct answer is option 'D'. Can you explain this answer?
At 273 K and 1 atm, 1 L of N2O4 (g) decomposes to NO2(g)a s given,
At equilibrium, original volume is 25% less than the existing volume. Percentage decomposition of N2O4 (g) is thus,
a)
25%
b)
50%
c)
66.66%
d)
33.33%
|
|
Suresh Reddy answered |
Let the initial volume of N2O4 be x and initial volume of NO2 is 0
If the degree of dissociation is a, then the final volume of N2O4 is x(1−a) and NO2 is 2ax.
Initial
It equilibrium
N2O4 ⟶ 2NO2
x 0
x(1−a) 2ax
Total initial volume =x+0=x
Final volume =x(1−a)+2ax=x+ax=x(1+a)
It is given that the initial volume is 25% less than the final volume
x=0.75×(1+a)
1+a=1.33
a=0.33
So %age dissociation = 33.33%
If the degree of dissociation is a, then the final volume of N2O4 is x(1−a) and NO2 is 2ax.
Initial
It equilibrium
N2O4 ⟶ 2NO2
x 0
x(1−a) 2ax
Total initial volume =x+0=x
Final volume =x(1−a)+2ax=x+ax=x(1+a)
It is given that the initial volume is 25% less than the final volume
x=0.75×(1+a)
1+a=1.33
a=0.33
So %age dissociation = 33.33%
Direction (Q. Nos. 16-19) This section contains a paragraph, each describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)Passage I
The equilibrium reaction 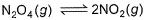 has been thoroughly studied Kp = 0.148 at 298 KIf the total pressure in a flask containing NO2 and N2O4 gas at 25°C is 1.50 atm, what fraction of the N2O4 has dissociated to NO2 ?
has been thoroughly studied Kp = 0.148 at 298 KIf the total pressure in a flask containing NO2 and N2O4 gas at 25°C is 1.50 atm, what fraction of the N2O4 has dissociated to NO2 ? - a)0.156
- b)0.844
- c)0.024
- d)0.076
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 16-19) This section contains a paragraph, each describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
Passage I
The equilibrium reaction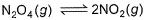 has been thoroughly studied Kp = 0.148 at 298 K
has been thoroughly studied Kp = 0.148 at 298 K
The equilibrium reaction
If the total pressure in a flask containing NO2 and N2O4 gas at 25°C is 1.50 atm, what fraction of the N2O4 has dissociated to NO2 ?
a)
0.156
b)
0.844
c)
0.024
d)
0.076
|
|
Suresh Iyer answered |
The correct answer is Option A.
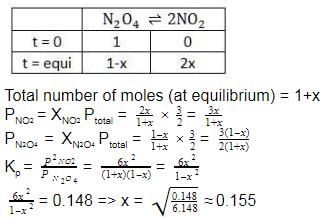
Fraction of N2O4 dissociated = x = 0.155 (x = mole fraction)
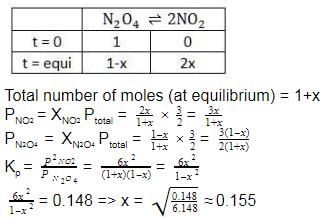
Fraction of N2O4 dissociated = x = 0.155 (x = mole fraction)
Passage lIOne of the reactions that takes place in producing steel from iron ore is the reduction of iron (II) oxide by carbon monoxide to give iron metal and carbon dioxide.
Initial partial pressure
CO(g) = 1.40 atm
CO2(g) = 0.80 atmQ. Under the given partial pressure, reaction is- a)displaced in forward side
- b)displaced in backward side
- c)in equilibrium
- d)incomplete in the absence of total pressure
Correct answer is option 'C'. Can you explain this answer?
Passage lI
One of the reactions that takes place in producing steel from iron ore is the reduction of iron (II) oxide by carbon monoxide to give iron metal and carbon dioxide.
Initial partial pressure
CO(g) = 1.40 atm
CO2(g) = 0.80 atm
Initial partial pressure
CO(g) = 1.40 atm
CO2(g) = 0.80 atm
Q. Under the given partial pressure, reaction is
a)
displaced in forward side
b)
displaced in backward side
c)
in equilibrium
d)
incomplete in the absence of total pressure
|
|
Preeti Iyer answered |
We know that with increase in pressure on one side, reaction shifts to that side which has less no. of moles(of gaseous species). However the no. of moles are same on both sides. So the REACTION WILL REMAIN IN EQUILIBRIUM.
Ammonium carbamate dissociates as,
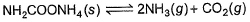
In a closed vessel containing ammonium carbamate in equilibrium with its vapour, ammonia is added such that partial pressure of NH3 now equals the original total pressure. Thus, ratio of the total pressure to the original pressure is- a)2:1
- b)31:27
- c)27 : 37
- d)
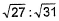
Correct answer is option 'B'. Can you explain this answer?
Ammonium carbamate dissociates as,
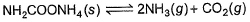
In a closed vessel containing ammonium carbamate in equilibrium with its vapour, ammonia is added such that partial pressure of NH3 now equals the original total pressure. Thus, ratio of the total pressure to the original pressure is
In a closed vessel containing ammonium carbamate in equilibrium with its vapour, ammonia is added such that partial pressure of NH3 now equals the original total pressure. Thus, ratio of the total pressure to the original pressure is
a)
2:1
b)
31:27
c)
27 : 37
d)

|
Imk Pathsala answered |
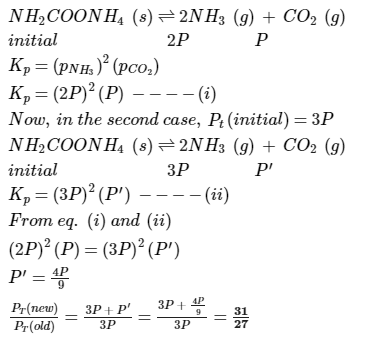
The effect of temperature on reaction rate is given by- a)Gibb’s Helmholtz equation
- b)Clausius Claperon equation
- c)Arrhenius equation
- d)Kirchoff’s equation
Correct answer is option 'C'. Can you explain this answer?
The effect of temperature on reaction rate is given by
a)
Gibb’s Helmholtz equation
b)
Clausius Claperon equation
c)
Arrhenius equation
d)
Kirchoff’s equation
|
|
Manoj Chauhan answered |
Arrhenius equation describes the effect of temperature on the rate constant of a chemical reaction. It is given by:
k = Ae^(-Ea/RT)
where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature in Kelvin.
Explanation:
Temperature is a measure of the average kinetic energy of the molecules in a substance. When the temperature is increased, the molecules move faster and collide more frequently. This increases the chance of successful collisions between reactant molecules, leading to an increase in the reaction rate.
Arrhenius equation explains this relationship between temperature and reaction rate by stating that the rate constant (k) of a reaction increases exponentially with increasing temperature. The activation energy (Ea) is the minimum energy required for a reaction to occur, and it determines the rate at which the reaction proceeds.
The pre-exponential factor (A) takes into account the frequency of collisions between reactant molecules. It is a constant that depends on the nature of the reaction and the reactants involved.
The Arrhenius equation is widely used to describe the temperature dependence of chemical reactions in various fields, including chemistry, biology, and materials science.
Conclusion:
In summary, the Arrhenius equation is used to describe the effect of temperature on the rate constant of a chemical reaction. It explains that the rate constant increases exponentially with increasing temperature, due to an increase in the frequency and energy of collisions between reactant molecules.
k = Ae^(-Ea/RT)
where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature in Kelvin.
Explanation:
Temperature is a measure of the average kinetic energy of the molecules in a substance. When the temperature is increased, the molecules move faster and collide more frequently. This increases the chance of successful collisions between reactant molecules, leading to an increase in the reaction rate.
Arrhenius equation explains this relationship between temperature and reaction rate by stating that the rate constant (k) of a reaction increases exponentially with increasing temperature. The activation energy (Ea) is the minimum energy required for a reaction to occur, and it determines the rate at which the reaction proceeds.
The pre-exponential factor (A) takes into account the frequency of collisions between reactant molecules. It is a constant that depends on the nature of the reaction and the reactants involved.
The Arrhenius equation is widely used to describe the temperature dependence of chemical reactions in various fields, including chemistry, biology, and materials science.
Conclusion:
In summary, the Arrhenius equation is used to describe the effect of temperature on the rate constant of a chemical reaction. It explains that the rate constant increases exponentially with increasing temperature, due to an increase in the frequency and energy of collisions between reactant molecules.
Rate of ionic reactions are generally- a)Very slow
- b)Very fast
- c)Slow
- d)Moderate
Correct answer is option 'B'. Can you explain this answer?
Rate of ionic reactions are generally
a)
Very slow
b)
Very fast
c)
Slow
d)
Moderate

|
Aryan Sen answered |
Ionic reactions occur very fast.
The ionic reactions are generally very fast because- a)Reactions are highly exothermic
- b)It does not involve bond breaking
- c)The energy of interaction is between charged ion is greater than between neutral molecules
- d)The number of collisions between ions are very large
Correct answer is option 'B'. Can you explain this answer?
The ionic reactions are generally very fast because
a)
Reactions are highly exothermic
b)
It does not involve bond breaking
c)
The energy of interaction is between charged ion is greater than between neutral molecules
d)
The number of collisions between ions are very large

|
Athul Patel answered |
Ionic reactions does not involve bond breaking therefore they are fast.
Passage IIA 15 L flask at 300 K contains 64.4 g of a mixture of NO2 and N2O4 in equilibrium. Given,
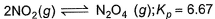 Q. Kc for the above equilibrium is
Q. Kc for the above equilibrium is - a)164.28
- b) 6.087x 10-3
- c)0.2708
- d)3.693
Correct answer is option 'A'. Can you explain this answer?
Passage II
A 15 L flask at 300 K contains 64.4 g of a mixture of NO2 and N2O4 in equilibrium. Given,
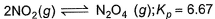
Q. Kc for the above equilibrium is
a)
164.28
b)
6.087x 10-3
c)
0.2708
d)
3.693
|
|
Pooja Shah answered |
Kp = Kc(RT)∆n
Kp = 6.67 ,
∆n = moles of products - moles of reactants = 1-2 = -1
R = 0.0821 L atm mol-¹K-¹
T = 300K
∆n = moles of products - moles of reactants = 1-2 = -1
R = 0.0821 L atm mol-¹K-¹
T = 300K
Substitute these values in the formula,
=> Kc = 6.67×0.0821×300
Kc = 164.28.
=> Kc = 6.67×0.0821×300
Kc = 164.28.
For the first order reaction, half life is equal to- a)
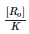
- b)
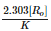
- c)
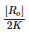
- d)0.693/K
Correct answer is option 'D'. Can you explain this answer?
For the first order reaction, half life is equal to
a)
b)
c)
d)
0.693/K
|
|
Om Desai answered |
For 1st order reaction half life is independent of initial concentration of reactant.
The half life of a reaction is halved as the initial concentration of the reactant is doubled. The order of the reaction is- a)2
- b)0
- c)1
- d)3
Correct answer is option 'A'. Can you explain this answer?
The half life of a reaction is halved as the initial concentration of the reactant is doubled. The order of the reaction is
a)
2
b)
0
c)
1
d)
3
|
|
Snehal Iyer answered |
Explanation:
The half-life of a reaction is defined as the time required for the concentration of a reactant to decrease by half.
Let's assume that the initial concentration of a reactant is [A] and its half-life is t1/2.
When the initial concentration of the reactant is doubled, the new concentration becomes 2[A]. Let's assume that the new half-life is t1/2'.
Now, we know that the rate of a reaction is directly proportional to the concentration of the reactant raised to the power of the order of the reaction.
Thus, we can write the following equation for the rate constant (k) of the reaction:
k = A*[A]^n
where A is a proportionality constant and n is the order of the reaction.
Now, let's compare the rate constants of the reaction at two different concentrations.
When the initial concentration of the reactant is [A], the rate constant is:
k = A*[A]^n
When the initial concentration of the reactant is 2[A], the rate constant is:
k' = A*[2A]^n = 2^n*A*[A]^n
We know that the half-life of the reaction is inversely proportional to the rate constant. Thus, we can write the following equation for the half-life of the reaction:
t1/2 = ln(2)/(k*[A]^n)
t1/2' = ln(2)/(k'*[2A]^n) = ln(2)/(2^n*A*[A]^n)
Now, let's compare the two half-lives.
t1/2' = ln(2)/(2^n*A*[A]^n) = (1/2)*ln(2)/(A*[A]^n)
We know that t1/2' is half of t1/2.
Thus, we can write the following equation:
t1/2'/t1/2 = 1/2
(1/2)*ln(2)/(A*[A]^n)/(ln(2)/(A*[A]^n)) = 1/2
2^n = 2
n = 2
Thus, the order of the reaction is 2.
The half-life of a reaction is defined as the time required for the concentration of a reactant to decrease by half.
Let's assume that the initial concentration of a reactant is [A] and its half-life is t1/2.
When the initial concentration of the reactant is doubled, the new concentration becomes 2[A]. Let's assume that the new half-life is t1/2'.
Now, we know that the rate of a reaction is directly proportional to the concentration of the reactant raised to the power of the order of the reaction.
Thus, we can write the following equation for the rate constant (k) of the reaction:
k = A*[A]^n
where A is a proportionality constant and n is the order of the reaction.
Now, let's compare the rate constants of the reaction at two different concentrations.
When the initial concentration of the reactant is [A], the rate constant is:
k = A*[A]^n
When the initial concentration of the reactant is 2[A], the rate constant is:
k' = A*[2A]^n = 2^n*A*[A]^n
We know that the half-life of the reaction is inversely proportional to the rate constant. Thus, we can write the following equation for the half-life of the reaction:
t1/2 = ln(2)/(k*[A]^n)
t1/2' = ln(2)/(k'*[2A]^n) = ln(2)/(2^n*A*[A]^n)
Now, let's compare the two half-lives.
t1/2' = ln(2)/(2^n*A*[A]^n) = (1/2)*ln(2)/(A*[A]^n)
We know that t1/2' is half of t1/2.
Thus, we can write the following equation:
t1/2'/t1/2 = 1/2
(1/2)*ln(2)/(A*[A]^n)/(ln(2)/(A*[A]^n)) = 1/2
2^n = 2
n = 2
Thus, the order of the reaction is 2.
H2S (g) initially at a pressure of 10.0 atm and a temperature of 800 K, dissociates as
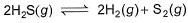 At equilibrium, the partial pressure of S2 vapour is 0.020 atm . Thus, Kp is
At equilibrium, the partial pressure of S2 vapour is 0.020 atm . Thus, Kp is - a)3.23x 10-7
- b)6.45x 10-7
- c)1.55x 106
- d)6.20x 107
Correct answer is option 'A'. Can you explain this answer?
H2S (g) initially at a pressure of 10.0 atm and a temperature of 800 K, dissociates as
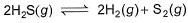
At equilibrium, the partial pressure of S2 vapour is 0.020 atm . Thus, Kp is
a)
3.23x 10-7
b)
6.45x 10-7
c)
1.55x 106
d)
6.20x 107
|
|
Geetika Shah answered |
The correct answer is Option A.
2H2S(g) ⇌ 2H2(g) + S2(g)
Pressure
at t=0 Pi − −
at eqm Pi−P 2P P
2H2S(g) ⇌ 2H2(g) + S2(g)
Pressure
at t=0 Pi − −
at eqm Pi−P 2P P
as P=0.02 thus Pi−P=10−0.02
Pi=10 2P=0.04

Kp = 3.23×10−7 atm.
Pi=10 2P=0.04

Kp = 3.23×10−7 atm.
Once the equilibrium is reached under given condition:- a)Cone, remains the same in spite of the change in temperature
- b)Cone, of all the substances presents do not change
- c)Cone, of reactants remairfs same
- d)Cone, of products remains same
Correct answer is option 'B'. Can you explain this answer?
Once the equilibrium is reached under given condition:
a)
Cone, remains the same in spite of the change in temperature
b)
Cone, of all the substances presents do not change
c)
Cone, of reactants remairfs same
d)
Cone, of products remains same
|
|
Raghav Bansal answered |
In a chemical reaction, chemical equilibrium is the state in which the forward reaction rate and the reverse reaction rate are equal. The result of this equilibrium is that the concentrations of the reactants and the products do not change. However, just because concentrations aren’t changing does not mean that all chemical reaction has ceased. Just the opposite is true; chemical equilibrium is a dynamic state in which reactants are being converted into products at all times, but at the exact rate that products are being converted back into reactants. The result of such a situation is analogous to a bridge between two cities, where the rate of cars going over the bridge in each direction is exactly equal. The result is that the net number of cars on either side of the bridge does not change.
The constant k used in rate equation is known as- a)Distance constant
- b)Velocity constant
- c)Reaction constant
- d)Order constant
Correct answer is option 'B'. Can you explain this answer?
The constant k used in rate equation is known as
a)
Distance constant
b)
Velocity constant
c)
Reaction constant
d)
Order constant
|
|
Nisha Kulkarni answered |
Rate Equation and Velocity Constant
Rate equation
The rate equation is an equation that describes the relationship between the rate of a chemical reaction and the concentration of the reactants. The rate of a reaction is the speed at which the reactants are converted into products.
The rate equation is expressed in the following form:
Rate = k[A]^m[B]^n
Where,
k = velocity constant or rate constant
[A] = concentration of reactant A
[B] = concentration of reactant B
m and n = order of the reaction with respect to A and B respectively.
Velocity constant
The velocity constant (k) is a proportionality constant that relates the rate of a reaction to the concentration of the reactants. It is also known as the rate constant.
The velocity constant is specific to a particular chemical reaction and is determined experimentally. It is a measure of the intrinsic tendency of the reactants to react and the efficiency of the reaction.
The value of the velocity constant depends on the temperature, pressure, and other conditions of the reaction. It is expressed in units of concentration per unit time.
Importance of velocity constant
The velocity constant is important in understanding the kinetics of a chemical reaction. It provides information about the rate of the reaction and the mechanism by which it occurs.
The value of the velocity constant can be used to predict the rate of the reaction at different concentrations of the reactants. It can also be used to compare the rates of different reactions and to optimize reaction conditions.
Conclusion
In conclusion, the velocity constant (k) is an important parameter in the rate equation that relates the rate of a chemical reaction to the concentration of the reactants. It is specific to a particular reaction and is determined experimentally. The value of the velocity constant provides information about the kinetics of the reaction and can be used to predict the rate of the reaction at different concentrations of the reactants.
Rate equation
The rate equation is an equation that describes the relationship between the rate of a chemical reaction and the concentration of the reactants. The rate of a reaction is the speed at which the reactants are converted into products.
The rate equation is expressed in the following form:
Rate = k[A]^m[B]^n
Where,
k = velocity constant or rate constant
[A] = concentration of reactant A
[B] = concentration of reactant B
m and n = order of the reaction with respect to A and B respectively.
Velocity constant
The velocity constant (k) is a proportionality constant that relates the rate of a reaction to the concentration of the reactants. It is also known as the rate constant.
The velocity constant is specific to a particular chemical reaction and is determined experimentally. It is a measure of the intrinsic tendency of the reactants to react and the efficiency of the reaction.
The value of the velocity constant depends on the temperature, pressure, and other conditions of the reaction. It is expressed in units of concentration per unit time.
Importance of velocity constant
The velocity constant is important in understanding the kinetics of a chemical reaction. It provides information about the rate of the reaction and the mechanism by which it occurs.
The value of the velocity constant can be used to predict the rate of the reaction at different concentrations of the reactants. It can also be used to compare the rates of different reactions and to optimize reaction conditions.
Conclusion
In conclusion, the velocity constant (k) is an important parameter in the rate equation that relates the rate of a chemical reaction to the concentration of the reactants. It is specific to a particular reaction and is determined experimentally. The value of the velocity constant provides information about the kinetics of the reaction and can be used to predict the rate of the reaction at different concentrations of the reactants.
The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is called- a)Threshold energy
- b)Potential energy
- c)Internal energy
- d)Activation energy
Correct answer is option 'D'. Can you explain this answer?
The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is called
a)
Threshold energy
b)
Potential energy
c)
Internal energy
d)
Activation energy

|
Ciel Knowledge answered |
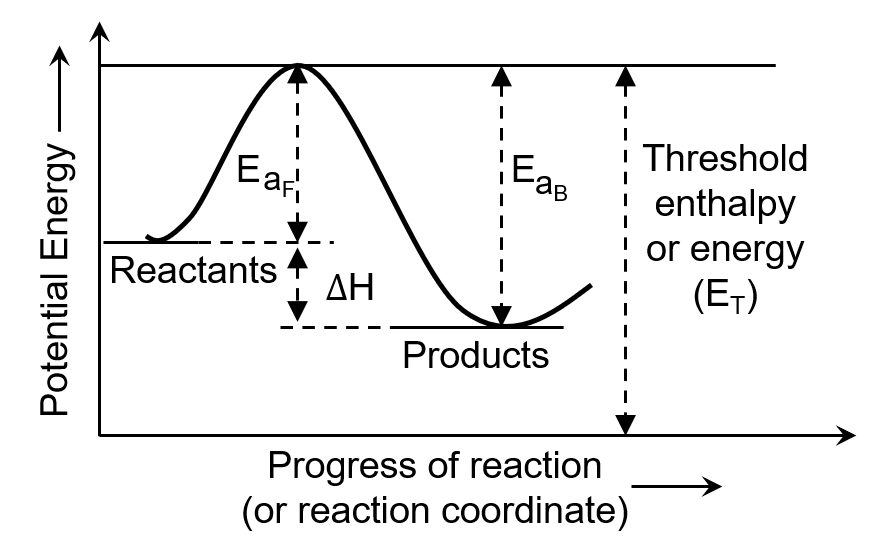
The minimum energy that the colliding molecules must possess for the chemical reaction to occur is known as threshold energy.
The extra energy required by a reactant to participate in a reaction is called activation energy.
The rate law for the reaction 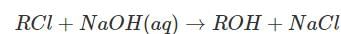 is given by rate = k[RCl]. The rate for this reaction
is given by rate = k[RCl]. The rate for this reaction
- a)is doubled by doubling the concentration of NaOH
- b)is halved by doubling the concentration of NaOH
- c)is unaffected by change in temperature
- d)is halved by half by reducing the concentration of RCl
Correct answer is option 'D'. Can you explain this answer?
The rate law for the reaction 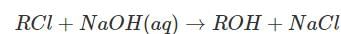 is given by rate = k[RCl]. The rate for this reaction
is given by rate = k[RCl]. The rate for this reaction
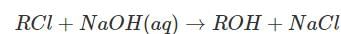 is given by rate = k[RCl]. The rate for this reaction
is given by rate = k[RCl]. The rate for this reactiona)
is doubled by doubling the concentration of NaOH
b)
is halved by doubling the concentration of NaOH
c)
is unaffected by change in temperature
d)
is halved by half by reducing the concentration of RCl
|
|
Tanuja Kapoor answered |
Since order of reaction with respect to RCl is one so if conc of RCl is halfed the rate of reaction will also become half.
Chapter doubts & questions for Rate Processes – Kinetics and Equilibrium (GC) - MCAT Chemical and Physical Foundations 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Rate Processes – Kinetics and Equilibrium (GC) - MCAT Chemical and Physical Foundations in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
MCAT Chemical and Physical Foundations
336 videos|223 docs|109 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily