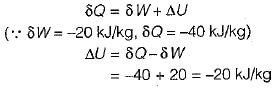All Exams >
Mechanical Engineering >
Topicwise Question Bank for Mechanical Engineering >
All Questions
All questions of Thermodynamics for Mechanical Engineering Exam
The net work done for the closed system shown in the given pressure-volume diagram is
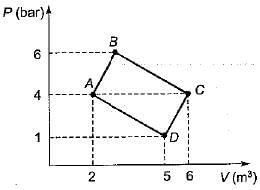
- a)600 kN-m
- b)1000 kN-m
- c)900 kN-m
- d)700kN-m
Correct answer is option 'B'. Can you explain this answer?
The net work done for the closed system shown in the given pressure-volume diagram is


a)
600 kN-m
b)
1000 kN-m
c)
900 kN-m
d)
700kN-m

|
Manish Aggarwal answered |
Correct Answer :- B
Explanation : AC = 4 m3.
ABC = 2, ACD = 3
Work done = Area of ABC + Area of ACD
= 1/2 * 2 * 4 + 1/2 * 3 * 4
= 1000 kN-m.
Which of the following second-law statements is incorrect?
- a)The entropy of an isolated system must remain constant or increase.
- b)The entropy of a hot copper block decreases as it cools.
- c)If ice is melted in water in an insulated container, the net entropy decreases.
- d)Work must be input if energy is transferred from a cold body to a hot body.
Correct answer is option 'C'. Can you explain this answer?
Which of the following second-law statements is incorrect?
a)
The entropy of an isolated system must remain constant or increase.
b)
The entropy of a hot copper block decreases as it cools.
c)
If ice is melted in water in an insulated container, the net entropy decreases.
d)
Work must be input if energy is transferred from a cold body to a hot body.

|
Tanishq Rane answered |
Incorrect Second-Law Statement:
The incorrect second-law statement is option 'C': If ice is melted in water in an insulated container, the net entropy decreases.
Explanation:
The second law of thermodynamics states that the total entropy of an isolated system can never decrease over time. Entropy is a measure of the disorder or randomness of a system. Hence, the correct statement is that the entropy of an isolated system must remain constant or increase.
When ice is melted in water in an insulated container, the net entropy of the system increases. Although the ice melts and becomes more disordered, the water molecules become more ordered and lose some entropy. However, the increase in entropy due to the melting of ice is much greater than the decrease in entropy due to the ordering of water molecules. Therefore, the net effect is an increase in entropy.
Conclusion:
Option 'C' is an incorrect statement because it violates the second law of thermodynamics. The correct statement is that the net entropy of an isolated system can never decrease over time.
The incorrect second-law statement is option 'C': If ice is melted in water in an insulated container, the net entropy decreases.
Explanation:
The second law of thermodynamics states that the total entropy of an isolated system can never decrease over time. Entropy is a measure of the disorder or randomness of a system. Hence, the correct statement is that the entropy of an isolated system must remain constant or increase.
When ice is melted in water in an insulated container, the net entropy of the system increases. Although the ice melts and becomes more disordered, the water molecules become more ordered and lose some entropy. However, the increase in entropy due to the melting of ice is much greater than the decrease in entropy due to the ordering of water molecules. Therefore, the net effect is an increase in entropy.
Conclusion:
Option 'C' is an incorrect statement because it violates the second law of thermodynamics. The correct statement is that the net entropy of an isolated system can never decrease over time.
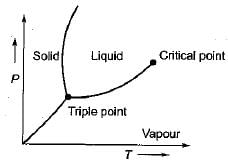
A substance expands on freezing only if
- a)the slope of fusion line on p-T chart is - ve
- b)the slope of sublimation line on p- T chart is +ve
- c)the slope of sublimation line on p-T chart is -ve
- d)the slope of fusion line on p-T chart is +ve
Correct answer is option 'A'. Can you explain this answer?
A substance expands on freezing only if
a)
the slope of fusion line on p-T chart is - ve
b)
the slope of sublimation line on p- T chart is +ve
c)
the slope of sublimation line on p-T chart is -ve
d)
the slope of fusion line on p-T chart is +ve
|
|
Yash Das answered |
Correct Answer :- A
Explanation : A substance expands on freezing only if the slope of the fusion line on the pressure temperature chart is negative.
A stationary mass of gas is compressed without friction from an initial state of 0.3 m3 and 1 MPa to a final state of 0.15 m3 and 1 MPa, the pressure remaining constant during the process. There is a transfer of 40 kJ of heat from the gas during the process. What is the change in internal energy of the gas?- a)-5 kJ
- b)+25 kJ
- c)-25 kJ
- d)+15 kJ
Correct answer is option 'C'. Can you explain this answer?
A stationary mass of gas is compressed without friction from an initial state of 0.3 m3 and 1 MPa to a final state of 0.15 m3 and 1 MPa, the pressure remaining constant during the process. There is a transfer of 40 kJ of heat from the gas during the process. What is the change in internal energy of the gas?
a)
-5 kJ
b)
+25 kJ
c)
-25 kJ
d)
+15 kJ
|
|
Shruti Bose answered |
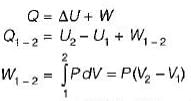
= 0.1 χ 106 (0.15-0.3)
= -15Kj
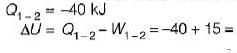 -25Kj
-25KjIn a Carnot engine, when working substance gives heat to the sink- a)temperature of sink increases
- b)temperature of sink remains same
- c)temperature of source decreases
- d)temperature of both the sink and source decreases
Correct answer is option 'B'. Can you explain this answer?
In a Carnot engine, when working substance gives heat to the sink
a)
temperature of sink increases
b)
temperature of sink remains same
c)
temperature of source decreases
d)
temperature of both the sink and source decreases
|
|
Yash Patel answered |
In Carnot engine, source and sink is thermal energy reservoir hence as heat is rejected or extracted, temperature does not change.
Which of the following is an example of heterogeneous system?- a)Atmospheric air
- b)Mixture of hydrogen and oxygen
- c)Cooling fluid in a radiator
- d)Mixture of ice, water and steam
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an example of heterogeneous system?
a)
Atmospheric air
b)
Mixture of hydrogen and oxygen
c)
Cooling fluid in a radiator
d)
Mixture of ice, water and steam

|
Prerna Menon answered |
Heterogeneous System:
A heterogeneous system refers to a mixture or combination of different substances that are not uniform in composition or properties. In such systems, the individual components can be distinguished and separated from each other by physical means.
Explanation:
Among the given options, the correct example of a heterogeneous system is the mixture of ice, water, and steam (option D). Let's understand why this is the case:
1. Mixture of ice, water, and steam:
- This system consists of three different phases of water: solid ice, liquid water, and gaseous steam.
- Each phase has distinct physical properties, such as density, temperature, and state of matter.
- These components can be visually observed and separated from each other by physical means.
- For example, if we have a container with ice, water, and steam, we can easily separate them by applying heat. The steam will evaporate, the water will remain in liquid form, and the ice will melt into water.
- The components in this system do not have a uniform composition throughout, making it a heterogeneous system.
2. Other options:
- Atmospheric air (option A) is a homogeneous system as it is a mixture of gases (mainly nitrogen, oxygen, carbon dioxide, etc.), which are uniformly distributed throughout the air. The composition of gases remains relatively constant.
- A mixture of hydrogen and oxygen (option B) can be either a homogeneous or heterogeneous system depending on the proportions. If it is a well-mixed mixture, it can be considered homogeneous. However, if the gases are not uniformly mixed, it can be considered heterogeneous.
- Cooling fluid in a radiator (option C) can also be considered a homogeneous system as it typically consists of a well-mixed liquid coolant, such as water or a specialized coolant mixture.
Conclusion:
Among the given options, the mixture of ice, water, and steam (option D) is an example of a heterogeneous system. The components in this system (ice, water, and steam) have different physical properties and can be visually distinguished and separated from each other.
A heterogeneous system refers to a mixture or combination of different substances that are not uniform in composition or properties. In such systems, the individual components can be distinguished and separated from each other by physical means.
Explanation:
Among the given options, the correct example of a heterogeneous system is the mixture of ice, water, and steam (option D). Let's understand why this is the case:
1. Mixture of ice, water, and steam:
- This system consists of three different phases of water: solid ice, liquid water, and gaseous steam.
- Each phase has distinct physical properties, such as density, temperature, and state of matter.
- These components can be visually observed and separated from each other by physical means.
- For example, if we have a container with ice, water, and steam, we can easily separate them by applying heat. The steam will evaporate, the water will remain in liquid form, and the ice will melt into water.
- The components in this system do not have a uniform composition throughout, making it a heterogeneous system.
2. Other options:
- Atmospheric air (option A) is a homogeneous system as it is a mixture of gases (mainly nitrogen, oxygen, carbon dioxide, etc.), which are uniformly distributed throughout the air. The composition of gases remains relatively constant.
- A mixture of hydrogen and oxygen (option B) can be either a homogeneous or heterogeneous system depending on the proportions. If it is a well-mixed mixture, it can be considered homogeneous. However, if the gases are not uniformly mixed, it can be considered heterogeneous.
- Cooling fluid in a radiator (option C) can also be considered a homogeneous system as it typically consists of a well-mixed liquid coolant, such as water or a specialized coolant mixture.
Conclusion:
Among the given options, the mixture of ice, water, and steam (option D) is an example of a heterogeneous system. The components in this system (ice, water, and steam) have different physical properties and can be visually distinguished and separated from each other.
The following data refer to a 6-cylinder, single- acting, two-stroke diesel engine:
Speed-250 rpm
Cylinder diameter - 0.7 m
Stroke of a piston - 1.2 m
Area of indicator diagram - 5.5 x 10-4 m2
Length of diagram - 0.06 m
Spring value -144 MPa per mWhat is the net rate of work transfer from the gas to the pistons in kW?- a)11434 ,
- b)20000
- c)15232
- d)17323
Correct answer is option 'C'. Can you explain this answer?
The following data refer to a 6-cylinder, single- acting, two-stroke diesel engine:
Speed-250 rpm
Cylinder diameter - 0.7 m
Stroke of a piston - 1.2 m
Area of indicator diagram - 5.5 x 10-4 m2
Length of diagram - 0.06 m
Spring value -144 MPa per m
Speed-250 rpm
Cylinder diameter - 0.7 m
Stroke of a piston - 1.2 m
Area of indicator diagram - 5.5 x 10-4 m2
Length of diagram - 0.06 m
Spring value -144 MPa per m
What is the net rate of work transfer from the gas to the pistons in kW?
a)
11434 ,
b)
20000
c)
15232
d)
17323
|
|
Lavanya Menon answered |
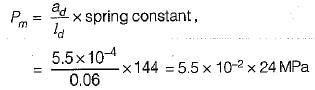
Net work transfer,
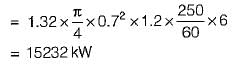
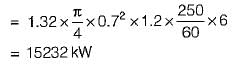
A piston-cylinder device contains a liquid-vapor mixture of water at 300 K. During a constant- pressure process, 750 kJ of heat is transferred to the water. As a result, part of the liquid in the cylinder vaporizes. What is entropy change of the water during this process?- a)-1.5 kJ/K
- b)1.5kJ/K
- c)2.5 kJ/K
- d)- 2 .5 k J/K .
Correct answer is option 'C'. Can you explain this answer?
A piston-cylinder device contains a liquid-vapor mixture of water at 300 K. During a constant- pressure process, 750 kJ of heat is transferred to the water. As a result, part of the liquid in the cylinder vaporizes. What is entropy change of the water during this process?
a)
-1.5 kJ/K
b)
1.5kJ/K
c)
2.5 kJ/K
d)
- 2 .5 k J/K .
|
|
Yash Patel answered |
The system undergoes an internally reversible, isothermal process, and thus its entropy change,

A balloon containing an ideal gas is initially kept in an evacuated and insulated room. The balloon ruptures and the gas fills up the entire room. Which one of the following statement is true at the end of the above process?- a)The internal energy of the gas decreases from its initial value, but the enthalpy remains constant
- b)The internal energy of the gas increases from its initial value, but the enthalpy remains constant
- c)Both internal energy and enthalpy of the gas remains constant
- d)Both internal energy and the enthalpy of the gas increase
Correct answer is option 'C'. Can you explain this answer?
A balloon containing an ideal gas is initially kept in an evacuated and insulated room. The balloon ruptures and the gas fills up the entire room. Which one of the following statement is true at the end of the above process?
a)
The internal energy of the gas decreases from its initial value, but the enthalpy remains constant
b)
The internal energy of the gas increases from its initial value, but the enthalpy remains constant
c)
Both internal energy and enthalpy of the gas remains constant
d)
Both internal energy and the enthalpy of the gas increase

|
Maulik Joshi answered |
Understanding the Scenario
When the balloon containing an ideal gas ruptures in an insulated and evacuated room, the gas expands to fill the available volume. This process involves specific thermodynamic principles that govern the behavior of ideal gases.
Key Principles Involved
- Insulated System: The room is insulated, meaning no heat can enter or leave the system. This leads to adiabatic conditions.
- Ideal Gas Behavior: For an ideal gas, internal energy (U) is primarily a function of temperature (T). Enthalpy (H) is related to internal energy and pressure-volume work.
Internal Energy Changes
- Internal Energy Remains Constant: Since the gas expands into a vacuum (no external work done), and the process is adiabatic, there is no heat transfer. Therefore, the internal energy of the gas does not change.
Enthalpy Considerations
- Enthalpy Remains Constant: Under the conditions of constant temperature and pressure (which is true as the gas expands to fill the room without doing work), the enthalpy also remains unchanged.
Conclusion
In this specific scenario, both internal energy and enthalpy of the gas remain constant after the gas fills the room. Thus, the correct answer is option 'C': Both internal energy and the enthalpy of the gas remain constant.
This is a fundamental outcome of the principles governing ideal gases in adiabatic processes within insulated systems.
When the balloon containing an ideal gas ruptures in an insulated and evacuated room, the gas expands to fill the available volume. This process involves specific thermodynamic principles that govern the behavior of ideal gases.
Key Principles Involved
- Insulated System: The room is insulated, meaning no heat can enter or leave the system. This leads to adiabatic conditions.
- Ideal Gas Behavior: For an ideal gas, internal energy (U) is primarily a function of temperature (T). Enthalpy (H) is related to internal energy and pressure-volume work.
Internal Energy Changes
- Internal Energy Remains Constant: Since the gas expands into a vacuum (no external work done), and the process is adiabatic, there is no heat transfer. Therefore, the internal energy of the gas does not change.
Enthalpy Considerations
- Enthalpy Remains Constant: Under the conditions of constant temperature and pressure (which is true as the gas expands to fill the room without doing work), the enthalpy also remains unchanged.
Conclusion
In this specific scenario, both internal energy and enthalpy of the gas remain constant after the gas fills the room. Thus, the correct answer is option 'C': Both internal energy and the enthalpy of the gas remain constant.
This is a fundamental outcome of the principles governing ideal gases in adiabatic processes within insulated systems.
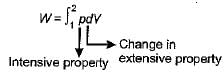
Consider the following:
1. Temperature
2. Viscosity
3. Specific entropy
4. Thermal conductivityWhich of the above properties of a system is/are intensive- a)1 only
- b)2 and 3 only
- c)2, 3 and 4 only
- d)1 and 2 only
Correct answer is option 'D'. Can you explain this answer?
Consider the following:
1. Temperature
2. Viscosity
3. Specific entropy
4. Thermal conductivity
1. Temperature
2. Viscosity
3. Specific entropy
4. Thermal conductivity
Which of the above properties of a system is/are intensive
a)
1 only
b)
2 and 3 only
c)
2, 3 and 4 only
d)
1 and 2 only
|
|
Amrita Chauhan answered |
Intensive and Extensive Properties:
In thermodynamics, properties of a system can be classified as either intensive or extensive. Intensive properties are independent of the size or amount of the system, while extensive properties depend on the size or amount of the system.
Explanation of the given properties:
1. Entropy:
Entropy is a measure of the disorder or randomness of a system. It is a state function and is independent of the size or amount of the system. Therefore, entropy is an intensive property.
2. Viscosity:
Viscosity is a measure of a fluid's resistance to flow. It is determined by the internal friction between the fluid molecules. Viscosity is independent of the size or amount of the fluid and is therefore an intensive property.
3. Temperature:
Temperature is a measure of the average kinetic energy of the particles in a system. It is independent of the size or amount of the system and is therefore an intensive property.
4. Specific heat at constant volume:
Specific heat at constant volume is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius at constant volume. It is a material-specific property and is independent of the size or amount of the substance. Therefore, specific heat at constant volume is an intensive property.
Conclusion:
From the explanations above, we can conclude that options 'a', 'b', 'c' are incorrect as they do not include all the intensive properties. Option 'D' is the correct answer as it includes all the given properties (viscosity, temperature, and specific heat at constant volume) that are intensive properties.
In thermodynamics, properties of a system can be classified as either intensive or extensive. Intensive properties are independent of the size or amount of the system, while extensive properties depend on the size or amount of the system.
Explanation of the given properties:
1. Entropy:
Entropy is a measure of the disorder or randomness of a system. It is a state function and is independent of the size or amount of the system. Therefore, entropy is an intensive property.
2. Viscosity:
Viscosity is a measure of a fluid's resistance to flow. It is determined by the internal friction between the fluid molecules. Viscosity is independent of the size or amount of the fluid and is therefore an intensive property.
3. Temperature:
Temperature is a measure of the average kinetic energy of the particles in a system. It is independent of the size or amount of the system and is therefore an intensive property.
4. Specific heat at constant volume:
Specific heat at constant volume is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius at constant volume. It is a material-specific property and is independent of the size or amount of the substance. Therefore, specific heat at constant volume is an intensive property.
Conclusion:
From the explanations above, we can conclude that options 'a', 'b', 'c' are incorrect as they do not include all the intensive properties. Option 'D' is the correct answer as it includes all the given properties (viscosity, temperature, and specific heat at constant volume) that are intensive properties.
Energy is added to 5 kg of air with a paddle wheel so that ΔT = 100°C while P = const. in an insulated container. The paddle-wheel work is- a)502.5 kJ
- b)482 kJ
- c)412 kJ
- d)358 kJ
Correct answer is option 'D'. Can you explain this answer?
Energy is added to 5 kg of air with a paddle wheel so that ΔT = 100°C while P = const. in an insulated container. The paddle-wheel work is
a)
502.5 kJ
b)
482 kJ
c)
412 kJ
d)
358 kJ

|
Constructing Careers answered |
To determine the paddle-wheel work done on 5 kg of air with a temperature increase of 100°C at constant pressure in an insulated container, we use thermodynamic principles.
- Identify the Process:
- Since the container is insulated, there's no heat transfer (Q = 0).
- The first law of thermodynamics simplifies to ΔU = W, where W is the work done by the paddle wheel.
- Internal Energy Change Calculation:
- Air can be treated as an ideal diatomic gas.
- Specific heat at constant volume for a diatomic gas, Cv = (5/2)R = 20.785 J/mol·K.
- Molar mass of air ≈ 28.97 g/mol → Moles of air, n = 5000 g / 28.97 g/mol ≈ 172.6 mol.
- ΔU = n × Cv × ΔT = 172.6 × 20.785 J/K × 100 K ≈ 358,800 J or 358.8 kJ.
- Conclusion: The work done by the paddle wheel equals the internal energy change (ΔU), resulting in approximately 358 kJ.
Thus, the correct answer is option D: 358 kJ.
If a heat engine attains 100% thermal efficiency, it violates- a)zeroth law of thermodynamics
- b)first law of thermodynamics
- c)second law of thermodynamics
- d)third law of thermodynamic
Correct answer is option 'C'. Can you explain this answer?
If a heat engine attains 100% thermal efficiency, it violates
a)
zeroth law of thermodynamics
b)
first law of thermodynamics
c)
second law of thermodynamics
d)
third law of thermodynamic
|
|
Sarita Yadav answered |
Such a heat engine is PMM2 which is impossible. It violates kelvin-Plank statement
A diathermic wall is one which- a)prevents thermal interaction
- b)permits thermal interaction
- c)encourages thermal interaction
- d)discourages thermal interaction
Correct answer is option 'B'. Can you explain this answer?
A diathermic wall is one which
a)
prevents thermal interaction
b)
permits thermal interaction
c)
encourages thermal interaction
d)
discourages thermal interaction

|
Jay Menon answered |
Diathermic Wall in Thermal Interaction
Definition: A diathermic wall is a type of wall that permits thermal interaction between two or more systems.
Explanation:
Thermal interaction refers to the exchange of thermal energy between two or more systems. A diathermic wall is a type of wall that allows this exchange of thermal energy to occur. The wall is said to be diathermic because it is permeable to heat transfer. In other words, heat can flow through the wall and be exchanged between the systems on either side of the wall.
A diathermic wall is commonly used in many engineering applications where thermal interaction between different systems is required. For instance, in a heat exchanger, a diathermic wall separates the two fluids that are being exchanged. The wall allows heat to flow between the fluids, thus facilitating the transfer of thermal energy from one fluid to the other.
Examples:
Some common examples of diathermic walls include:
- The walls of a furnace that allow heat to pass from the furnace to the surrounding environment.
- The walls of a heat exchanger that allow thermal energy to transfer between two or more fluids.
- The walls of a building that allow heat to flow from the inside to the outside or vice versa.
- The walls of a refrigerator that allow heat to flow from the inside to the outside.
Conclusion:
In summary, a diathermic wall is a type of wall that permits thermal interaction between two or more systems. The wall allows heat to flow through it and be exchanged between the systems on either side of the wall. Diathermic walls are commonly used in many engineering applications where thermal interaction is required, such as in heat exchangers, furnaces, and buildings.
Definition: A diathermic wall is a type of wall that permits thermal interaction between two or more systems.
Explanation:
Thermal interaction refers to the exchange of thermal energy between two or more systems. A diathermic wall is a type of wall that allows this exchange of thermal energy to occur. The wall is said to be diathermic because it is permeable to heat transfer. In other words, heat can flow through the wall and be exchanged between the systems on either side of the wall.
A diathermic wall is commonly used in many engineering applications where thermal interaction between different systems is required. For instance, in a heat exchanger, a diathermic wall separates the two fluids that are being exchanged. The wall allows heat to flow between the fluids, thus facilitating the transfer of thermal energy from one fluid to the other.
Examples:
Some common examples of diathermic walls include:
- The walls of a furnace that allow heat to pass from the furnace to the surrounding environment.
- The walls of a heat exchanger that allow thermal energy to transfer between two or more fluids.
- The walls of a building that allow heat to flow from the inside to the outside or vice versa.
- The walls of a refrigerator that allow heat to flow from the inside to the outside.
Conclusion:
In summary, a diathermic wall is a type of wall that permits thermal interaction between two or more systems. The wall allows heat to flow through it and be exchanged between the systems on either side of the wall. Diathermic walls are commonly used in many engineering applications where thermal interaction is required, such as in heat exchangers, furnaces, and buildings.
The more effective way of increasing the efficiency of a Carnot engine is to- a)increase higher temperature
- b)decrease higher temperature
- c)increase lower temperature
- d)decrease lower temperature
Correct answer is option 'D'. Can you explain this answer?
The more effective way of increasing the efficiency of a Carnot engine is to
a)
increase higher temperature
b)
decrease higher temperature
c)
increase lower temperature
d)
decrease lower temperature
|
|
Rhea Reddy answered |
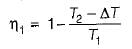
(decreasing lower temperature by ΔT)
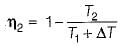
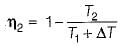
(increasing higher temperature by ΔT)
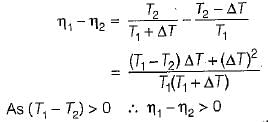
So, the more effective way to increase the cycle efficiency is to decrease lower temperature.
The maximum amount of mechanical energy that can be converted into heat in any process- a)depends on source and sink temperature
- b)depends on friction present
- c)depends on nature of mechanical energy
- d)is 100%
Correct answer is option 'D'. Can you explain this answer?
The maximum amount of mechanical energy that can be converted into heat in any process
a)
depends on source and sink temperature
b)
depends on friction present
c)
depends on nature of mechanical energy
d)
is 100%

|
Sanaya Sengupta answered |
Since mechanical energy is high grade energy and heat is low grade energy, 100% conversion of high grade energy into low grade energy is possible.
For an isolated system executing a process
1. no heat transfer takes place
2. no work is done
3. no mass crosses the boundary
4. no chemical reaction takes place within the systemWhich of the above statement are correct?- a)1,2 and 3
- b)1,3 and 4
- c)2, 3 and 4
- d)all of the above
Correct answer is option 'A'. Can you explain this answer?
For an isolated system executing a process
1. no heat transfer takes place
2. no work is done
3. no mass crosses the boundary
4. no chemical reaction takes place within the system
1. no heat transfer takes place
2. no work is done
3. no mass crosses the boundary
4. no chemical reaction takes place within the system
Which of the above statement are correct?
a)
1,2 and 3
b)
1,3 and 4
c)
2, 3 and 4
d)
all of the above
|
|
Gaurav Kapoor answered |
Isolated System and its characteristics
An isolated system is a thermodynamic system that does not exchange energy or matter with its surroundings. The system is completely self-contained, and there is no interaction with the environment. The system can be a physical or theoretical construct, and it is often used in thermodynamics to simplify the analysis of a process or system.
Characteristics of an isolated system:
- No heat transfer takes place: In an isolated system, there is no transfer of heat energy between the system and its surroundings. The system is thermally insulated, and the temperature of the system remains constant.
- No work is done: An isolated system does not perform any work on its surroundings, nor is any work done on the system by the surroundings.
- No mass crosses the boundary: In an isolated system, there is no transfer of matter across the system boundary. The total mass of the system remains constant.
- No chemical reaction takes place within the system: An isolated system does not undergo any chemical reactions within the system.
Correct statement for an isolated system
From the given options, the correct statement for an isolated system is:
a) 1, 2, and 3
Explanation:
- No heat transfer takes place: As an isolated system is thermally insulated, there is no transfer of heat between the system and its surroundings.
- No work is done: An isolated system does not perform any work on its surroundings or vice versa. Hence, no work is done.
- No mass crosses the boundary: In an isolated system, there is no transfer of matter across the system boundary. Hence, the total mass of the system remains constant.
Therefore, option 'a' is the correct statement for an isolated system.
An isolated system is a thermodynamic system that does not exchange energy or matter with its surroundings. The system is completely self-contained, and there is no interaction with the environment. The system can be a physical or theoretical construct, and it is often used in thermodynamics to simplify the analysis of a process or system.
Characteristics of an isolated system:
- No heat transfer takes place: In an isolated system, there is no transfer of heat energy between the system and its surroundings. The system is thermally insulated, and the temperature of the system remains constant.
- No work is done: An isolated system does not perform any work on its surroundings, nor is any work done on the system by the surroundings.
- No mass crosses the boundary: In an isolated system, there is no transfer of matter across the system boundary. The total mass of the system remains constant.
- No chemical reaction takes place within the system: An isolated system does not undergo any chemical reactions within the system.
Correct statement for an isolated system
From the given options, the correct statement for an isolated system is:
a) 1, 2, and 3
Explanation:
- No heat transfer takes place: As an isolated system is thermally insulated, there is no transfer of heat between the system and its surroundings.
- No work is done: An isolated system does not perform any work on its surroundings or vice versa. Hence, no work is done.
- No mass crosses the boundary: In an isolated system, there is no transfer of matter across the system boundary. Hence, the total mass of the system remains constant.
Therefore, option 'a' is the correct statement for an isolated system.
Variation of pressure and volume at constant temperature are correlated through- a)Charle’s law
- b)Boyle’s law
- c)Joule’s law
- d)Gay Lussac’s law
Correct answer is option 'B'. Can you explain this answer?
Variation of pressure and volume at constant temperature are correlated through
a)
Charle’s law
b)
Boyle’s law
c)
Joule’s law
d)
Gay Lussac’s law

|
Pallabi Tiwari answered |
As per Boyle’s law
At constant temperature
PV = Constant
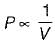
At constant temperature
PV = Constant
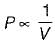
The following heat engine produces power of 100,000 kW. The heat engine operates between 800 K and 300 K. It has a thermal efficiency equal to 50% of that of the Carnot engine for the same temperatures. The rate at which heat is absorbed from the hot reservoir is- a)100 MW
- b)160 MW
- c)200 MW
- d)320 M
Correct answer is option 'D'. Can you explain this answer?
The following heat engine produces power of 100,000 kW. The heat engine operates between 800 K and 300 K. It has a thermal efficiency equal to 50% of that of the Carnot engine for the same temperatures. The rate at which heat is absorbed from the hot reservoir is
a)
100 MW
b)
160 MW
c)
200 MW
d)
320 M
|
|
Sandeep Sengupta answered |
Power produced by engine = Workdone(W) = 100,000 k
 = 50% of Carnot cycle or engine
= 50% of Carnot cycle or engine
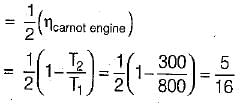
Also,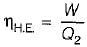
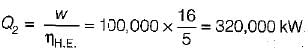
= 320 MW
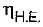 = 50% of Carnot cycle or engine
= 50% of Carnot cycle or engine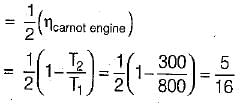
Also,
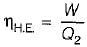
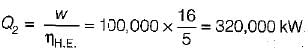
= 320 MW
Four process of thermodynamic cycle are shown in figure on P-V diagram in the sequence 1 -2-3-4. The corresponding correct sequence of these process in the T-S plane shown in figure will be
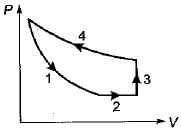
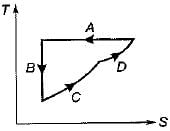
- a)A-B-C-D
- b)B-C-D-A
- c)C-D-A-B
- d)C-B-A-D
Correct answer is option 'B'. Can you explain this answer?
Four process of thermodynamic cycle are shown in figure on P-V diagram in the sequence 1 -2-3-4. The corresponding correct sequence of these process in the T-S plane shown in figure will be




a)
A-B-C-D
b)
B-C-D-A
c)
C-D-A-B
d)
C-B-A-D

|
Vishal Kushwaha answered |
B
A closed system undergoes a process 1-2 for which the values of Q1-2 and W1-2 are +20 kJ and +50 kJ respectively. If the system is returned to state 1 and Q2-1 is - 10 kJ what is the value of work W2-1- a)+20 kJ
- b)-40 kJ
- c)-80 kJ
- d)+40 kJ
Correct answer is option 'B'. Can you explain this answer?
A closed system undergoes a process 1-2 for which the values of Q1-2 and W1-2 are +20 kJ and +50 kJ respectively. If the system is returned to state 1 and Q2-1 is - 10 kJ what is the value of work W2-1
a)
+20 kJ
b)
-40 kJ
c)
-80 kJ
d)
+40 kJ
|
|
Jhanvi Datta answered |
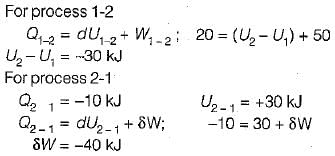
Zeroth law of thermodynamics states that:- a)two thermodynamic systems are always in thermal equilibrium with each other.
- b)if two systems are in thermal equilibrium, then the third system will also be in thermal equilibrium.
- c)two systems not in thermal equilibrium with a third system are also not in thermal equilibrium with each other.
- d)when two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
Correct answer is option 'D'. Can you explain this answer?
Zeroth law of thermodynamics states that:
a)
two thermodynamic systems are always in thermal equilibrium with each other.
b)
if two systems are in thermal equilibrium, then the third system will also be in thermal equilibrium.
c)
two systems not in thermal equilibrium with a third system are also not in thermal equilibrium with each other.
d)
when two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
|
|
Zoya Sharma answered |
The zeroth law of thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other. ... This says in essence that the three bodies are all the same temperature.
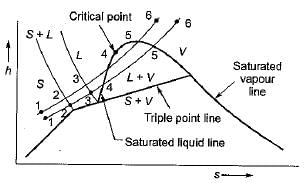
Which one of the following represents the condensation of a mixture of saturated liquid and saturated vapour on the enthalpy-entropy diagram- a)a horizontal line
- b)an inclined line of constant slope
- c)a vertical line
- d)a curved line
Correct answer is option 'B'. Can you explain this answer?
Which one of the following represents the condensation of a mixture of saturated liquid and saturated vapour on the enthalpy-entropy diagram
a)
a horizontal line
b)
an inclined line of constant slope
c)
a vertical line
d)
a curved line

|
Shalini Deshpande answered |
For Moiller diagram, condensation takes place at constant pressure which is along vertically inclined.
If steam is throttled its- a)pressure and enthalpy remain unchanged
- b)temperature and entropy remain unchanged
- c)enthalpy remains unchanged but the other property change
- d)enthalpy remains unchanged but pressure may or may not chan
Correct answer is option 'C'. Can you explain this answer?
If steam is throttled its
a)
pressure and enthalpy remain unchanged
b)
temperature and entropy remain unchanged
c)
enthalpy remains unchanged but the other property change
d)
enthalpy remains unchanged but pressure may or may not chan

|
Rajat Patel answered |
If steam is throttled , its enthalpy remains constant and pressure drop takes place
A heat engine receives heat from a source at 1500 K at a rate of 600 kW and rejects the waste heat to a medium at 350 K. If the power output from the heat engine is 250 kW, the irreversibility rate for the process is- a)125 kW
- b)150 kW
- c)180 kW
- d)210 kW
Correct answer is option 'D'. Can you explain this answer?
A heat engine receives heat from a source at 1500 K at a rate of 600 kW and rejects the waste heat to a medium at 350 K. If the power output from the heat engine is 250 kW, the irreversibility rate for the process is
a)
125 kW
b)
150 kW
c)
180 kW
d)
210 kW
|
|
Rashi Chauhan answered |
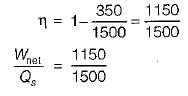
Work is done on a adiabatic system due to which its velocity changes from 10 m/s to 20 m/s, elevation
increases by 20 m and temperature increases by 1 K. The mass of the system is 10 kg, Cv = 100 J/(kg.K) and gravitational acceleration is 10 m/s2. If there is no change in any other component of the energy of the system, the magnitude of total work done (in kJ) on the system is Correct answer is '4.5'. Can you explain this answer?
Work is done on a adiabatic system due to which its velocity changes from 10 m/s to 20 m/s, elevation
increases by 20 m and temperature increases by 1 K. The mass of the system is 10 kg, Cv = 100 J/(kg.K) and gravitational acceleration is 10 m/s2. If there is no change in any other component of the energy of the system, the magnitude of total work done (in kJ) on the system is
increases by 20 m and temperature increases by 1 K. The mass of the system is 10 kg, Cv = 100 J/(kg.K) and gravitational acceleration is 10 m/s2. If there is no change in any other component of the energy of the system, the magnitude of total work done (in kJ) on the system is
|
|
Debolina Menon answered |
Understanding the Problem
In an adiabatic system, work is done, resulting in changes in velocity, elevation, and temperature. We need to calculate the total work done on the system with given parameters.
Given Data
- Initial velocity (u) = 10 m/s
- Final velocity (v) = 20 m/s
- Elevation increase (h) = 20 m
- Temperature increase (ΔT) = 1 K
- Mass (m) = 10 kg
- Specific heat at constant volume (Cv) = 100 J/(kg·K)
- Gravitational acceleration (g) = 10 m/s²
Calculating Work Done
1. Change in Kinetic Energy (KE):
- KE_initial = 0.5 * m * u² = 0.5 * 10 * (10)² = 500 J
- KE_final = 0.5 * m * v² = 0.5 * 10 * (20)² = 2000 J
- Change in KE = KE_final - KE_initial = 2000 J - 500 J = 1500 J
2. Change in Potential Energy (PE):
- PE = m * g * h = 10 * 10 * 20 = 2000 J
3. Change in Internal Energy (UE):
- UE = m * Cv * ΔT = 10 * 100 * 1 = 1000 J
Total Energy Change
- Total Energy Change = Change in KE + Change in PE + Change in UE
- Total Energy Change = 1500 J + 2000 J + 1000 J = 4500 J
Calculating Total Work Done
- Since the system is adiabatic, the total work done (W) is equal to the total energy change.
- W = 4500 J = 4.5 kJ
Conclusion
The total work done on the system is 4.5 kJ, considering changes in kinetic energy, potential energy, and internal energy.
In an adiabatic system, work is done, resulting in changes in velocity, elevation, and temperature. We need to calculate the total work done on the system with given parameters.
Given Data
- Initial velocity (u) = 10 m/s
- Final velocity (v) = 20 m/s
- Elevation increase (h) = 20 m
- Temperature increase (ΔT) = 1 K
- Mass (m) = 10 kg
- Specific heat at constant volume (Cv) = 100 J/(kg·K)
- Gravitational acceleration (g) = 10 m/s²
Calculating Work Done
1. Change in Kinetic Energy (KE):
- KE_initial = 0.5 * m * u² = 0.5 * 10 * (10)² = 500 J
- KE_final = 0.5 * m * v² = 0.5 * 10 * (20)² = 2000 J
- Change in KE = KE_final - KE_initial = 2000 J - 500 J = 1500 J
2. Change in Potential Energy (PE):
- PE = m * g * h = 10 * 10 * 20 = 2000 J
3. Change in Internal Energy (UE):
- UE = m * Cv * ΔT = 10 * 100 * 1 = 1000 J
Total Energy Change
- Total Energy Change = Change in KE + Change in PE + Change in UE
- Total Energy Change = 1500 J + 2000 J + 1000 J = 4500 J
Calculating Total Work Done
- Since the system is adiabatic, the total work done (W) is equal to the total energy change.
- W = 4500 J = 4.5 kJ
Conclusion
The total work done on the system is 4.5 kJ, considering changes in kinetic energy, potential energy, and internal energy.
The equation, δQ = dU + PdV, is true for- a)any system, any process
- b)any system, reversible process
- c)closed system, any process
- d)closed system, only reversible process
Correct answer is option 'D'. Can you explain this answer?
The equation, δQ = dU + PdV, is true for
a)
any system, any process
b)
any system, reversible process
c)
closed system, any process
d)
closed system, only reversible process

|
Isha Bajaj answered |
Usually written as y = mx + b, represents a linear equation. The variables in this equation are y and x, which represent the dependent and independent variables, respectively. The coefficient m represents the slope of the line, which determines the steepness of the line. The term b represents the y-intercept, which is the point where the line crosses the y-axis. By plugging in different values for x, you can find corresponding values for y and plot those points on a graph to create a line.
In a quasiequilibrium process, the pressure in a system
- a)unconstant
- b)varies with temperature
- c)constant at any given instant throughout the system
- d)increase if volume increases
Correct answer is option 'C'. Can you explain this answer?
In a quasiequilibrium process, the pressure in a system
a)
unconstant
b)
varies with temperature
c)
constant at any given instant throughout the system
d)
increase if volume increases
|
|
Meera Bose answered |
Explanation:
Quasiequilibrium process is a process that occurs slowly enough for the system to remain in thermal and mechanical equilibrium at all times. In such a process, the pressure within the system is everywhere constant at an instant. This is because any variation in pressure within the system would cause the system to move towards a state of equilibrium, which is characterized by uniform pressure throughout the system. Therefore, in a quasiequilibrium process, the pressure remains constant throughout the system at any given instant.
Some important points to note about quasiequilibrium process are:
- It is a reversible process, meaning that it can be reversed by an infinitesimal change in the external conditions.
- It is a slow process, meaning that it occurs over a long period of time, allowing the system to remain in thermal and mechanical equilibrium at all times.
- It is an idealized process, meaning that it is not possible to achieve it in practice, but it provides a useful theoretical framework for analyzing real-world processes.
Conclusion:
In conclusion, in a quasiequilibrium process, the pressure within the system remains constant at any given instant. This is because the system is always in thermal and mechanical equilibrium, and any variation in pressure would cause the system to move towards a state of equilibrium characterized by uniform pressure throughout the system.
Quasiequilibrium process is a process that occurs slowly enough for the system to remain in thermal and mechanical equilibrium at all times. In such a process, the pressure within the system is everywhere constant at an instant. This is because any variation in pressure within the system would cause the system to move towards a state of equilibrium, which is characterized by uniform pressure throughout the system. Therefore, in a quasiequilibrium process, the pressure remains constant throughout the system at any given instant.
Some important points to note about quasiequilibrium process are:
- It is a reversible process, meaning that it can be reversed by an infinitesimal change in the external conditions.
- It is a slow process, meaning that it occurs over a long period of time, allowing the system to remain in thermal and mechanical equilibrium at all times.
- It is an idealized process, meaning that it is not possible to achieve it in practice, but it provides a useful theoretical framework for analyzing real-world processes.
Conclusion:
In conclusion, in a quasiequilibrium process, the pressure within the system remains constant at any given instant. This is because the system is always in thermal and mechanical equilibrium, and any variation in pressure would cause the system to move towards a state of equilibrium characterized by uniform pressure throughout the system.
Which among the following is not a boundary phenomenon?- a)Work transfer
- b)Heat transfer
- c)Mass transfer
- d)Change of temperature
Correct answer is option 'D'. Can you explain this answer?
Which among the following is not a boundary phenomenon?
a)
Work transfer
b)
Heat transfer
c)
Mass transfer
d)
Change of temperature
|
|
Nandita Chakraborty answered |
Not a Boundary Phenomenon:
- A boundary phenomenon is a physical process that occurs at the interface between two different systems or phases.
- It is a transfer of energy or matter across the boundary between the two systems.
- Among the options given, the one that is not a boundary phenomenon is a change of temperature.
Explanation:
- Work transfer, heat transfer, and mass transfer are all examples of boundary phenomena.
- Work transfer involves the transfer of energy across a boundary due to the application of a force.
- Heat transfer involves the transfer of thermal energy across a boundary due to a temperature difference.
- Mass transfer involves the transfer of matter across a boundary due to a concentration difference.
- A change of temperature, on the other hand, is not a transfer of energy or matter across a boundary. It is a change in the state of the system itself.
- Therefore, the correct answer is option D, change of temperature, which is not a boundary phenomenon.
- A boundary phenomenon is a physical process that occurs at the interface between two different systems or phases.
- It is a transfer of energy or matter across the boundary between the two systems.
- Among the options given, the one that is not a boundary phenomenon is a change of temperature.
Explanation:
- Work transfer, heat transfer, and mass transfer are all examples of boundary phenomena.
- Work transfer involves the transfer of energy across a boundary due to the application of a force.
- Heat transfer involves the transfer of thermal energy across a boundary due to a temperature difference.
- Mass transfer involves the transfer of matter across a boundary due to a concentration difference.
- A change of temperature, on the other hand, is not a transfer of energy or matter across a boundary. It is a change in the state of the system itself.
- Therefore, the correct answer is option D, change of temperature, which is not a boundary phenomenon.
Which one of the following statements applicable to a perfect gas will also be true for an irreversible process- a)δQ = dU + PdV
- b)δQ = TdS
- c)TdS = dU + PdV
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Which one of the following statements applicable to a perfect gas will also be true for an irreversible process
a)
δQ = dU + PdV
b)
δQ = TdS
c)
TdS = dU + PdV
d)
None of these

|
Niharika Yadav answered |
δQ = dU+ PdV... Applicable for a closed system when only PdV work is present. This is true only for a reversible process.
δQ = TdS ...Applicable for a reversible process.
TdS = dU + PdV... Applicable for any process reversible or irreversible, undergone by a closed system, since it is a relation among properties which are independent of the path.
δQ = TdS ...Applicable for a reversible process.
TdS = dU + PdV... Applicable for any process reversible or irreversible, undergone by a closed system, since it is a relation among properties which are independent of the path.
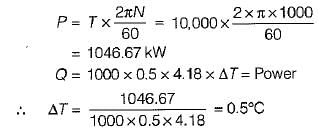
An engine is tested by means of a water brake at 1000 rpm. The measured torque-of the engine is 10000 Nm and the water consumption of the brake is 0.5 m3/s, its inlet temperature being 25°C. Assuming that the whole of the engine power is ultimately transferred into heat which is absorbed by the cooling water, what is the water temperature at exit?- a)22.5°C
- b)23.5°C
- c)24.5°C
- d)25.5°C
Correct answer is option 'D'. Can you explain this answer?
An engine is tested by means of a water brake at 1000 rpm. The measured torque-of the engine is 10000 Nm and the water consumption of the brake is 0.5 m3/s, its inlet temperature being 25°C. Assuming that the whole of the engine power is ultimately transferred into heat which is absorbed by the cooling water, what is the water temperature at exit?
a)
22.5°C
b)
23.5°C
c)
24.5°C
d)
25.5°C
|
|
Stuti Mishra answered |
Engine power,
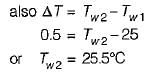
According to thermodynamics, the universe is defined as_______.
- a)System
- b)System + Surrounding
- c)Surrounding
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
According to thermodynamics, the universe is defined as_______.
a)
System
b)
System + Surrounding
c)
Surrounding
d)
None of these

|
Anjana Mukherjee answered |
In thermodynamics, a system is any part of the universe which is under observation and the rest of the universe, except the system, are called its surroundings.
So, universe = system + surroundings.
The integrating factor of quasi-static displacement work is- a)1/T
- b)1/P
- c)1/V
- d)P/T
Correct answer is option 'B'. Can you explain this answer?
The integrating factor of quasi-static displacement work is
a)
1/T
b)
1/P
c)
1/V
d)
P/T
|
|
Anshul Basu answered |
Quasistatic work,
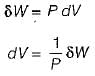
An inexact different dW when multiplied by an integrating factor 1/P becomes an exact differential dV.
(Point function) = (Integrating factor) x (Path function)
(Point function) = (Integrating factor) x (Path function)
300 kJ of heat is supplied at constant temperature of 500 K, to a heat engine. The heat rejection takes place at 300 K. The following result are obtained (a) 210 kJ (b) 180 kJ (c) 150 kJ. Identify whether result is a reversible cycle. Irreversible cycle or impossible cycle respectively
- a)reversible / irreversible / impossible respectively
- b)irreversible / reversible / impossible respectively
- c)irreversible / impossible / reversible respectively.
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
300 kJ of heat is supplied at constant temperature of 500 K, to a heat engine. The heat rejection takes place at 300 K. The following result are obtained (a) 210 kJ (b) 180 kJ (c) 150 kJ. Identify whether result is a reversible cycle. Irreversible cycle or impossible cycle respectively
a)
reversible / irreversible / impossible respectively
b)
irreversible / reversible / impossible respectively
c)
irreversible / impossible / reversible respectively.
d)
None of the above
|
|
Vibhor Goyal answered |


Match List-1 with List-11 and select the correct answer using the codes given below.
List - I
A. Work done
B. Thermal equilibrium
C. Internal energy
D. No work and heat interaction
List - II
1. Point function
2. Path function
3. Isolated system
4. Equality of temperature
Codes:
A B C D
(a) 2 4 1 3
(b) 2 3 4 2
(c) 3 1 2 4
(d) 4 2 3 1- a)(a)
- b)(b)
- c)(c)
- d)(d)
Correct answer is option 'A'. Can you explain this answer?
Match List-1 with List-11 and select the correct answer using the codes given below.
List - I
A. Work done
B. Thermal equilibrium
C. Internal energy
D. No work and heat interaction
List - II
1. Point function
2. Path function
3. Isolated system
4. Equality of temperature
Codes:
A B C D
(a) 2 4 1 3
(b) 2 3 4 2
(c) 3 1 2 4
(d) 4 2 3 1
List - I
A. Work done
B. Thermal equilibrium
C. Internal energy
D. No work and heat interaction
List - II
1. Point function
2. Path function
3. Isolated system
4. Equality of temperature
Codes:
A B C D
(a) 2 4 1 3
(b) 2 3 4 2
(c) 3 1 2 4
(d) 4 2 3 1
a)
(a)
b)
(b)
c)
(c)
d)
(d)

|
Srestha Khanna answered |

The variation of saturation pressure with saturation temperature for a liquid is 0.1 bar/K at 400 K. The specific volume of saturated liquid and dry saturated vapour at 400 K are 0.251 and 0.001 m3/kg. What will be value of latent heat of vaporization using Clausius clapeyron equation?- a)16000 kJ/kg
- b)1600 kJ/kg
- c)1000 kJ/kg
- d)160 kK/kg
Correct answer is option 'C'. Can you explain this answer?
The variation of saturation pressure with saturation temperature for a liquid is 0.1 bar/K at 400 K. The specific volume of saturated liquid and dry saturated vapour at 400 K are 0.251 and 0.001 m3/kg. What will be value of latent heat of vaporization using Clausius clapeyron equation?
a)
16000 kJ/kg
b)
1600 kJ/kg
c)
1000 kJ/kg
d)
160 kK/kg
|
|
Kritika Joshi answered |
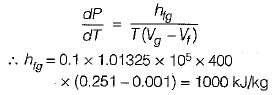
Rate of change of enthalpy with respect to entropy at constant pressure for a perfect gas- a)increases as T increases
- b)decreases asT increases
- c)is independent of T
- d)none of the above
Correct answer is option 'A'. Can you explain this answer?
Rate of change of enthalpy with respect to entropy at constant pressure for a perfect gas
a)
increases as T increases
b)
decreases asT increases
c)
is independent of T
d)
none of the above

|
Nishanth Banerjee answered |
Explanation:
Rate of Change of Enthalpy with Respect to Entropy:
- The rate of change of enthalpy with respect to entropy at constant pressure for a perfect gas is known as the temperature coefficient of enthalpy.
- This coefficient is represented as (∂H/∂S)P, where H is enthalpy, S is entropy, and P is pressure.
Relationship with Temperature:
- As temperature (T) increases, the rate of change of enthalpy with respect to entropy at constant pressure for a perfect gas increases.
- This relationship can be explained by the definition of entropy, which is a measure of the disorder or randomness of a system.
- As temperature increases, the randomness or disorder of the system also increases, leading to a higher rate of change of enthalpy with respect to entropy.
Conclusion:
- Therefore, the correct answer to the given question is option 'A' - the rate of change of enthalpy with respect to entropy at constant pressure for a perfect gas increases as temperature increases.
Rate of Change of Enthalpy with Respect to Entropy:
- The rate of change of enthalpy with respect to entropy at constant pressure for a perfect gas is known as the temperature coefficient of enthalpy.
- This coefficient is represented as (∂H/∂S)P, where H is enthalpy, S is entropy, and P is pressure.
Relationship with Temperature:
- As temperature (T) increases, the rate of change of enthalpy with respect to entropy at constant pressure for a perfect gas increases.
- This relationship can be explained by the definition of entropy, which is a measure of the disorder or randomness of a system.
- As temperature increases, the randomness or disorder of the system also increases, leading to a higher rate of change of enthalpy with respect to entropy.
Conclusion:
- Therefore, the correct answer to the given question is option 'A' - the rate of change of enthalpy with respect to entropy at constant pressure for a perfect gas increases as temperature increases.
The value of entropy at 0 K is taken as- a)1
- b)0
- c)-1
- d)any value
Correct answer is option 'B'. Can you explain this answer?
The value of entropy at 0 K is taken as
a)
1
b)
0
c)
-1
d)
any value
|
|
Anjali Shah answered |
S = K ln W
where, S is entropy
K is Boltzmann constant
W is thermodynamic probability
when W = 1, it represents the greatest order,
S = 0. This occurs only at T = 0 K. This is the Nernst-Simon statement of third law of themnodyanamics.
where, S is entropy
K is Boltzmann constant
W is thermodynamic probability
when W = 1, it represents the greatest order,
S = 0. This occurs only at T = 0 K. This is the Nernst-Simon statement of third law of themnodyanamics.
Perpetual motion machine of second kind violates the
- a)First law of thermodynamics
- b)Kelvin-Plank statement
- c)Clausius statement
- d)Third law of thermodynamics
Correct answer is option 'B'. Can you explain this answer?
Perpetual motion machine of second kind violates the
a)
First law of thermodynamics
b)
Kelvin-Plank statement
c)
Clausius statement
d)
Third law of thermodynamics
|
|
Meera Bose answered |
Perpetual motion machine of the second kind (PMM2): A fictitious machine which produces net work in a complete cycle by exchanging heat with only one reservoir is called the PMM2.
It violates the Kelvin plank statement.
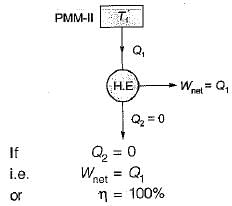
The heat engine will produce net work in a complete cycle by exchanging heat with only single reservoir thus violating the Kelvin-Piank statement.
At critical point the enthalpy of vapourization is- a)dependent on temperature only
- b)maximum
- c)minimum
- d)zero
Correct answer is option 'D'. Can you explain this answer?
At critical point the enthalpy of vapourization is
a)
dependent on temperature only
b)
maximum
c)
minimum
d)
zero

|
Jithin Choudhury answered |
At critical point enthalpy of vapourization is zero i.e. liquid can directly converted into vapour (without any latent heat transaction).
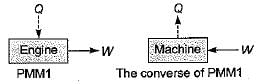
A PMM1 is- a)A thermodynamic machine
- b)A hypothetical machine
- c)A real machine
- d)A hypothetical machine whose operation would violate the first law of thermodynamic
Correct answer is option 'D'. Can you explain this answer?
A PMM1 is
a)
A thermodynamic machine
b)
A hypothetical machine
c)
A real machine
d)
A hypothetical machine whose operation would violate the first law of thermodynamic

|
Meghana Desai answered |
There can be no machine which would continuously supply mechanical work without some other form of energy disappearing simultaneously. It is fictitious machine.
Chapter doubts & questions for Thermodynamics - Topicwise Question Bank for Mechanical Engineering 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermodynamics - Topicwise Question Bank for Mechanical Engineering in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Topicwise Question Bank for Mechanical Engineering
45 videos|314 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup