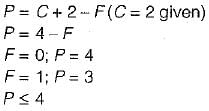All Exams >
Mechanical Engineering >
Engineering Materials >
All Questions
All questions of Phase Diagrams for Mechanical Engineering Exam
The percentage of pro-eutectoid ferrite and pearlite in a slowly cooled 0.5% carbon steel is- a)25% proeutectoid ferrite and 75% pearlite
- b)37.5% proeutectoid ferrite and 62.5% pearlite
- c)62.5% proeutectoid ferrite and 37.5% pearlite
- d)40% proeutectoid ferrite and 60% pearlite
Correct answer is option 'B'. Can you explain this answer?
The percentage of pro-eutectoid ferrite and pearlite in a slowly cooled 0.5% carbon steel is
a)
25% proeutectoid ferrite and 75% pearlite
b)
37.5% proeutectoid ferrite and 62.5% pearlite
c)
62.5% proeutectoid ferrite and 37.5% pearlite
d)
40% proeutectoid ferrite and 60% pearlite
|
|
Avinash Sharma answered |
Let fulcrum is at 0.5% carbon to apply lever rule. % Proeutectoid ferrite
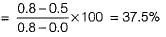
% Pearlite = 100 - 37.5 = 62.5%
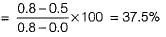
% Pearlite = 100 - 37.5 = 62.5%
Following is WRONG about a phase diagram:- a)It gives information on transformation rates.
- b)Relative amount of different phases can be found under given equilibrium conditions.
- c)It indicates the temperature at which different phases start to melt.
- d)Solid solubility limits are depicted by it.
Correct answer is option 'A'. Can you explain this answer?
Following is WRONG about a phase diagram:
a)
It gives information on transformation rates.
b)
Relative amount of different phases can be found under given equilibrium conditions.
c)
It indicates the temperature at which different phases start to melt.
d)
Solid solubility limits are depicted by it.

|
Ashwin Gupta answered |
Time dependence phase changes is studied by kinetics of the phase transformations.
If a particular Fe-C alloy contains less than 0.83% carbon. It is called
- a)hypoeutectoid steel
- b)hypereutectoid steel
- c)lhigh carbon steel
- d)cast iron
Correct answer is option 'A'. Can you explain this answer?
If a particular Fe-C alloy contains less than 0.83% carbon. It is called
a)
hypoeutectoid steel
b)
hypereutectoid steel
c)
lhigh carbon steel
d)
cast iron
|
|
Shruti Bose answered |
Pearlite is the eutectoid composition of ferrite and cementite in pearlite ferrite and cementite are found in alternate structure.
In which of the following phases of steel, cementite is in lamellar form?- a)Ferrite
- b)Bainite
- c)Martensite
- d)Pearlite
Correct answer is option 'D'. Can you explain this answer?
In which of the following phases of steel, cementite is in lamellar form?
a)
Ferrite
b)
Bainite
c)
Martensite
d)
Pearlite
|
|
Vaibhav Khanna answered |
Pearlite phase in steel is made up of alternate layers of ferrite and cementite.
Hardness of martensite is about- a)RC 65
- b)RC48
- c)RC 57
- d)RC 80
Correct answer is option 'A'. Can you explain this answer?
Hardness of martensite is about
a)
RC 65
b)
RC48
c)
RC 57
d)
RC 80
|
|
Akshara Rane answered |
0.2% C Martensite →50 RC
0.4% C Martensite →58 RC
0.8% C Martensite→ 65 RC
0.4% C Martensite →58 RC
0.8% C Martensite→ 65 RC
Free carbon in iron makes the iron
- a)soft and gives a fine crystalline structure
- b)soft and gives a coarse grained crystalline structure
- c)hard and gives a coarse grained crystalline structure
- d)hard and gives a fine grained crystalline structure
Correct answer is option 'B'. Can you explain this answer?
Free carbon in iron makes the iron
a)
soft and gives a fine crystalline structure
b)
soft and gives a coarse grained crystalline structure
c)
hard and gives a coarse grained crystalline structure
d)
hard and gives a fine grained crystalline structure
|
|
Sinjini Nambiar answered |
Hyper eutectoid steel ⇒ 0.76 to 2.1 percent carbon.
Which one of the following elements is a ferritic stabilizer?- a)Nickel
- b)Manganese
- c)Copper
- d)Chromium
Correct answer is option 'D'. Can you explain this answer?
Which one of the following elements is a ferritic stabilizer?
a)
Nickel
b)
Manganese
c)
Copper
d)
Chromium
|
|
Avantika Sen answered |
Chromium, Tungsten and Molybdenum are ferritic stabilizer.
Which one of the following sets of constituents is expected in equilibrium cooling of a hypereutectoid steel from austenitic state?- a)Ferrite and pearlite
- b)Cementite and pearlite
- c)Ferrite and bainite
- d)Cementite and martensite
Correct answer is option 'B'. Can you explain this answer?
Which one of the following sets of constituents is expected in equilibrium cooling of a hypereutectoid steel from austenitic state?
a)
Ferrite and pearlite
b)
Cementite and pearlite
c)
Ferrite and bainite
d)
Cementite and martensite
|
|
Shreya Choudhury answered |
Hypereutectoid steel when cooled in equilibrium will result in proeutectoid cementite and pearlite whereas hypoeutectoid steel when cooled in equilibrium will result in proeutectoid ferrite and pearlite.
The eutectoid of carbon in iron, above lower critical temperature, when cooled, result in- a)ferrite and austenite
- b)ferrite and cementite
- c)cementite and austenite
- d)ferrite, cementite and austenite
Correct answer is option 'B'. Can you explain this answer?
The eutectoid of carbon in iron, above lower critical temperature, when cooled, result in
a)
ferrite and austenite
b)
ferrite and cementite
c)
cementite and austenite
d)
ferrite, cementite and austenite
|
|
Neha Joshi answered |
The correct answer should be the second option - Ferrite and Cementite.
A eutectoid reaction is referred to the phase transformation or change of one solid into two other different solids.
Eutectoid reaction occurs at the eutectoid point of 727°C and 0.77% Carbon, which when cooled gives α-Ferrite and Cementite, also known as Pearlite. Below the critical temperature of 723°C, austenite is no more stable and it gets converted into pearlite.
A eutectoid reaction is referred to the phase transformation or change of one solid into two other different solids.
Eutectoid reaction occurs at the eutectoid point of 727°C and 0.77% Carbon, which when cooled gives α-Ferrite and Cementite, also known as Pearlite. Below the critical temperature of 723°C, austenite is no more stable and it gets converted into pearlite.
Eutectic product in Fe-C system is called- a)pearlite
- b)bainite
- c)ledeburite
- d)spheroidite
Correct answer is option 'C'. Can you explain this answer?
Eutectic product in Fe-C system is called
a)
pearlite
b)
bainite
c)
ledeburite
d)
spheroidite

|
Gowri Sharma answered |
A eutectic reaction occurs at 1146°C
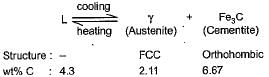
On cooling through the eutectic temperature, the lowest melting liquid of the system decomposes to solid phases, austenite and cementite. This eutectic mixture is knonw as ledeburite.

On cooling through the eutectic temperature, the lowest melting liquid of the system decomposes to solid phases, austenite and cementite. This eutectic mixture is knonw as ledeburite.
For good weldability, the carbon equivalent (%) of steel should be in the range of- a)0.2 - 0.4
- b)0.5 - 0.8
- c)0.7 - 0.8
- d)0.9 - 1.0
Correct answer is option 'A'. Can you explain this answer?
For good weldability, the carbon equivalent (%) of steel should be in the range of
a)
0.2 - 0.4
b)
0.5 - 0.8
c)
0.7 - 0.8
d)
0.9 - 1.0
|
|
Meera Bose answered |
Carbon Equivalent and Weldability of Steel
The carbon equivalent (CE) is a parameter used to measure the weldability of steel. Weldability refers to the ease with which a material can be welded without developing defects or experiencing problems during the welding process. The carbon equivalent value helps to predict the risk of cracking and other issues that may arise during welding.
Definition of Carbon Equivalent (CE)
The carbon equivalent is calculated based on the chemical composition of the steel, particularly the carbon content and the presence of other alloying elements. It is a numerical value that indicates the relative contribution of carbon and other elements to the weldability of the steel.
Range of Carbon Equivalent for Good Weldability
For good weldability, the carbon equivalent (%) of steel should be in the range of 0.2 - 0.4. This means that if the carbon equivalent value falls within this range, the steel is considered to have good weldability.
Explanation of the Correct Answer
The correct answer, option 'A' (0.2 - 0.4), is the range of carbon equivalent values that indicate good weldability. This range is widely accepted and used in various welding codes and standards.
Reason for the Range of 0.2 - 0.4
- Low Carbon Equivalent: A low carbon equivalent value indicates a low risk of cracking and other welding-related issues. This is because a lower carbon equivalent means a lower carbon content in the steel, which reduces the likelihood of hardening and cracking during welding.
- Optimum Carbon Equivalent: The range of 0.2 - 0.4 is considered the optimum range for good weldability. It strikes a balance between reducing the risk of welding defects and maintaining desirable mechanical properties in the welded joint.
- Effect of Alloying Elements: The presence of alloying elements such as manganese, silicon, and other elements affects the carbon equivalent value. These elements can help reduce the carbon equivalent and improve the weldability of the steel.
- Consideration of Welding Process: The carbon equivalent value is also influenced by the specific welding process being used. Some welding processes, such as high heat input processes, may require a lower carbon equivalent to ensure good weldability.
Conclusion
In summary, the carbon equivalent is an important parameter for assessing the weldability of steel. A carbon equivalent value in the range of 0.2 - 0.4 indicates good weldability, as it reduces the risk of welding defects while maintaining desirable mechanical properties in the welded joint. It is crucial to consider the carbon equivalent when selecting steel for welding applications to ensure successful and reliable welds.
The carbon equivalent (CE) is a parameter used to measure the weldability of steel. Weldability refers to the ease with which a material can be welded without developing defects or experiencing problems during the welding process. The carbon equivalent value helps to predict the risk of cracking and other issues that may arise during welding.
Definition of Carbon Equivalent (CE)
The carbon equivalent is calculated based on the chemical composition of the steel, particularly the carbon content and the presence of other alloying elements. It is a numerical value that indicates the relative contribution of carbon and other elements to the weldability of the steel.
Range of Carbon Equivalent for Good Weldability
For good weldability, the carbon equivalent (%) of steel should be in the range of 0.2 - 0.4. This means that if the carbon equivalent value falls within this range, the steel is considered to have good weldability.
Explanation of the Correct Answer
The correct answer, option 'A' (0.2 - 0.4), is the range of carbon equivalent values that indicate good weldability. This range is widely accepted and used in various welding codes and standards.
Reason for the Range of 0.2 - 0.4
- Low Carbon Equivalent: A low carbon equivalent value indicates a low risk of cracking and other welding-related issues. This is because a lower carbon equivalent means a lower carbon content in the steel, which reduces the likelihood of hardening and cracking during welding.
- Optimum Carbon Equivalent: The range of 0.2 - 0.4 is considered the optimum range for good weldability. It strikes a balance between reducing the risk of welding defects and maintaining desirable mechanical properties in the welded joint.
- Effect of Alloying Elements: The presence of alloying elements such as manganese, silicon, and other elements affects the carbon equivalent value. These elements can help reduce the carbon equivalent and improve the weldability of the steel.
- Consideration of Welding Process: The carbon equivalent value is also influenced by the specific welding process being used. Some welding processes, such as high heat input processes, may require a lower carbon equivalent to ensure good weldability.
Conclusion
In summary, the carbon equivalent is an important parameter for assessing the weldability of steel. A carbon equivalent value in the range of 0.2 - 0.4 indicates good weldability, as it reduces the risk of welding defects while maintaining desirable mechanical properties in the welded joint. It is crucial to consider the carbon equivalent when selecting steel for welding applications to ensure successful and reliable welds.
During peritectic solidification, one liquid
- a) solidifies into two different solids
- b)combine with one solid to form a second new solid
- c)forms one solid
- d)forms one solid and another liquid
Correct answer is option 'B'. Can you explain this answer?
During peritectic solidification, one liquid
a)
solidifies into two different solids
b)
combine with one solid to form a second new solid
c)
forms one solid
d)
forms one solid and another liquid

|
Sahana Chavan answered |
Peritectic Solidification
Peritectic solidification is a type of solidification process that occurs when a liquid phase reacts with a solid phase to form a new solid phase. This process is commonly observed in metal alloys, where the solidification behavior is influenced by the composition of the alloy and the cooling conditions.
Explanation of Option B
Option B states that during peritectic solidification, one liquid combines with one solid to form a second new solid. This statement accurately describes the peritectic solidification process.
Understanding Peritectic Solidification
To understand why option B is the correct answer, let's delve deeper into the peritectic solidification process. When a peritectic alloy undergoes cooling, the composition of the liquid phase changes, leading to the formation of a new solid phase.
The Peritectic Reaction
The peritectic reaction is the key mechanism behind peritectic solidification. It involves the transformation of a solid phase and a liquid phase into a new solid phase. The reaction can be represented as follows:
Solid (A) + Liquid (B) → Solid (C)
In this reaction, the solid phase A reacts with the liquid phase B to form a new solid phase C. The composition of the liquid phase B is critical in determining the characteristics of the newly formed solid phase C.
Formation of a Second New Solid
During peritectic solidification, the liquid phase combines with the existing solid phase to form a second new solid phase. This occurs because the composition of the liquid phase reaches a critical point where it can react with the solid phase to create a new solid phase. The new solid phase may have different properties and characteristics compared to the initial solid phase.
Example
For example, consider a peritectic alloy composed of elements A and B. As the alloy cools, the liquid phase with a specific composition combines with the solid phase A to form a second solid phase C. This new solid phase C may have a different crystal structure or composition compared to the initial solid phase A.
Conclusion
In summary, during peritectic solidification, one liquid combines with one solid to form a second new solid phase. This process is driven by the peritectic reaction, where the composition of the liquid phase reaches a critical point for reacting with the solid phase. Understanding peritectic solidification is crucial for predicting and controlling the solidification behavior of metal alloys.
Peritectic solidification is a type of solidification process that occurs when a liquid phase reacts with a solid phase to form a new solid phase. This process is commonly observed in metal alloys, where the solidification behavior is influenced by the composition of the alloy and the cooling conditions.
Explanation of Option B
Option B states that during peritectic solidification, one liquid combines with one solid to form a second new solid. This statement accurately describes the peritectic solidification process.
Understanding Peritectic Solidification
To understand why option B is the correct answer, let's delve deeper into the peritectic solidification process. When a peritectic alloy undergoes cooling, the composition of the liquid phase changes, leading to the formation of a new solid phase.
The Peritectic Reaction
The peritectic reaction is the key mechanism behind peritectic solidification. It involves the transformation of a solid phase and a liquid phase into a new solid phase. The reaction can be represented as follows:
Solid (A) + Liquid (B) → Solid (C)
In this reaction, the solid phase A reacts with the liquid phase B to form a new solid phase C. The composition of the liquid phase B is critical in determining the characteristics of the newly formed solid phase C.
Formation of a Second New Solid
During peritectic solidification, the liquid phase combines with the existing solid phase to form a second new solid phase. This occurs because the composition of the liquid phase reaches a critical point where it can react with the solid phase to create a new solid phase. The new solid phase may have different properties and characteristics compared to the initial solid phase.
Example
For example, consider a peritectic alloy composed of elements A and B. As the alloy cools, the liquid phase with a specific composition combines with the solid phase A to form a second solid phase C. This new solid phase C may have a different crystal structure or composition compared to the initial solid phase A.
Conclusion
In summary, during peritectic solidification, one liquid combines with one solid to form a second new solid phase. This process is driven by the peritectic reaction, where the composition of the liquid phase reaches a critical point for reacting with the solid phase. Understanding peritectic solidification is crucial for predicting and controlling the solidification behavior of metal alloys.
Eutectic composition of iron-carbon alloy always corresponds to its- a)lowest melting temperature
- b)highest melting temperature
- c)least carbon percentage
- d)highest fracture toughness
Correct answer is option 'D'. Can you explain this answer?
Eutectic composition of iron-carbon alloy always corresponds to its
a)
lowest melting temperature
b)
highest melting temperature
c)
least carbon percentage
d)
highest fracture toughness
|
|
Saikat Choudhary answered |
The eutectic composition of an iron-carbon alloy refers to the specific ratio of iron to carbon at which the alloy exhibits its lowest melting temperature. In other words, it is the composition at which the alloy has the highest amount of carbon that can be added without increasing the melting temperature. This composition is significant because it determines the microstructure and properties of the alloy, including its fracture toughness.
Explanation:
- **Eutectic Composition**: The eutectic composition is a specific ratio of iron to carbon in the alloy where the liquid phase transforms into a mixture of solid phases upon cooling, without any composition changes. In the case of iron-carbon alloys, the eutectic composition corresponds to approximately 4.3% carbon by weight. At this composition, the alloy exhibits its lowest melting temperature, which is around 1147 degrees Celsius.
- **Lowest Melting Temperature**: The eutectic composition has the lowest melting temperature because it represents a balanced ratio of iron and carbon atoms. At this composition, the carbon atoms have sufficient space to fit into the iron lattice structure, leading to the formation of a stable and low-energy microstructure. This allows for easier atomic movement and lower energy barriers for melting, resulting in a lower melting temperature.
- **Fracture Toughness**: Fracture toughness is a measure of a material's ability to resist crack propagation and withstand applied loads. It is influenced by various factors, including the microstructure of the material. In the case of iron-carbon alloys, the eutectic composition plays a role in determining the microstructure, which in turn affects the fracture toughness. The eutectic microstructure consists of alternating layers of ferrite and cementite, which provide a desirable combination of hardness and toughness. This microstructure enhances the material's resistance to crack propagation and improves its fracture toughness.
- **Highest Fracture Toughness**: Therefore, the eutectic composition of an iron-carbon alloy corresponds to the highest fracture toughness because it represents the optimal balance between hardness and toughness, resulting from the specific microstructure formed at this composition. Other compositions with higher or lower carbon percentages may exhibit different microstructures and, consequently, different fracture toughness values.
Explanation:
- **Eutectic Composition**: The eutectic composition is a specific ratio of iron to carbon in the alloy where the liquid phase transforms into a mixture of solid phases upon cooling, without any composition changes. In the case of iron-carbon alloys, the eutectic composition corresponds to approximately 4.3% carbon by weight. At this composition, the alloy exhibits its lowest melting temperature, which is around 1147 degrees Celsius.
- **Lowest Melting Temperature**: The eutectic composition has the lowest melting temperature because it represents a balanced ratio of iron and carbon atoms. At this composition, the carbon atoms have sufficient space to fit into the iron lattice structure, leading to the formation of a stable and low-energy microstructure. This allows for easier atomic movement and lower energy barriers for melting, resulting in a lower melting temperature.
- **Fracture Toughness**: Fracture toughness is a measure of a material's ability to resist crack propagation and withstand applied loads. It is influenced by various factors, including the microstructure of the material. In the case of iron-carbon alloys, the eutectic composition plays a role in determining the microstructure, which in turn affects the fracture toughness. The eutectic microstructure consists of alternating layers of ferrite and cementite, which provide a desirable combination of hardness and toughness. This microstructure enhances the material's resistance to crack propagation and improves its fracture toughness.
- **Highest Fracture Toughness**: Therefore, the eutectic composition of an iron-carbon alloy corresponds to the highest fracture toughness because it represents the optimal balance between hardness and toughness, resulting from the specific microstructure formed at this composition. Other compositions with higher or lower carbon percentages may exhibit different microstructures and, consequently, different fracture toughness values.
Seasonal cracking is observed in- a)Stainless steel
- b)Brass
- c)Cast iron
- d)Inconel
Correct answer is option 'B'. Can you explain this answer?
Seasonal cracking is observed in
a)
Stainless steel
b)
Brass
c)
Cast iron
d)
Inconel
|
|
Priyanka Tiwari answered |
Season cracking results from the combined effects of corrosion and internal stresses. The term seasonal cracking is usually applied to stress corrosion cracking of brass.
In which of the following phases'of steel cementite is in particle form?- a)Martensite
- b)Ferrite
- c)Pearlite
- d)Bainite
Correct answer is option 'D'. Can you explain this answer?
In which of the following phases'of steel cementite is in particle form?
a)
Martensite
b)
Ferrite
c)
Pearlite
d)
Bainite

|
Garima Basak answered |
Bainite: When austenite is cooled rapidly past the nose of C-curve and kept isothermally in a constant temperature bath, it transforms to a nonlamellar product known as bainite. It is an extremely fine mixture of ferrite and carbide. Bainite is present in two forms: the feathery bainite obtained in the upper part of temperature range and needie-like or accicular bainite produced by lower reaction temperature.
Increase of ferrite phase in steel increases- a)strength
- b)hardness
- c)ductility
- d)brittleness
Correct answer is option 'A'. Can you explain this answer?
Increase of ferrite phase in steel increases
a)
strength
b)
hardness
c)
ductility
d)
brittleness
|
|
Anu Deshpande answered |
Introduction:
The ferrite phase is a solid solution of carbon in iron. It has a body-centered cubic crystal structure and is relatively soft compared to other phases in steel. Increasing the amount of ferrite phase in steel can have several effects on its mechanical properties.
Strength:
Increasing the ferrite phase in steel can increase its strength. This is because ferrite has a lower carbon content, which reduces the formation of hard and brittle carbides. As a result, the steel becomes more ductile and less prone to fracture. The absence of carbides also reduces the likelihood of intergranular corrosion, which can further enhance the strength of the steel.
Hardness:
Contrary to the statement given in the question, an increase in the ferrite phase generally decreases the hardness of steel. Ferrite is a relatively soft phase, and increasing its proportion in steel leads to a decrease in overall hardness. Hardness is primarily determined by the presence of hard phases, such as martensite or carbides, which are not present in ferrite.
Ductility:
Increasing the ferrite phase in steel generally improves its ductility. Ferrite is a more ductile phase compared to other phases, such as martensite or bainite. The presence of ferrite allows for more plastic deformation before fracture occurs, making the steel more ductile and less prone to brittle failure.
Brittleness:
Increasing the ferrite phase in steel can reduce its brittleness. Ferrite is a relatively soft and ductile phase, and its presence can prevent the formation of harder and more brittle phases, such as martensite or bainite. This leads to a more ductile steel that is less prone to sudden and catastrophic failure.
Conclusion:
In summary, increasing the ferrite phase in steel can increase its strength, improve ductility, and reduce brittleness. However, it generally decreases the hardness of the steel. These changes in mechanical properties are primarily due to the absence of hard and brittle phases, such as carbides or martensite, in the ferrite phase.
The ferrite phase is a solid solution of carbon in iron. It has a body-centered cubic crystal structure and is relatively soft compared to other phases in steel. Increasing the amount of ferrite phase in steel can have several effects on its mechanical properties.
Strength:
Increasing the ferrite phase in steel can increase its strength. This is because ferrite has a lower carbon content, which reduces the formation of hard and brittle carbides. As a result, the steel becomes more ductile and less prone to fracture. The absence of carbides also reduces the likelihood of intergranular corrosion, which can further enhance the strength of the steel.
Hardness:
Contrary to the statement given in the question, an increase in the ferrite phase generally decreases the hardness of steel. Ferrite is a relatively soft phase, and increasing its proportion in steel leads to a decrease in overall hardness. Hardness is primarily determined by the presence of hard phases, such as martensite or carbides, which are not present in ferrite.
Ductility:
Increasing the ferrite phase in steel generally improves its ductility. Ferrite is a more ductile phase compared to other phases, such as martensite or bainite. The presence of ferrite allows for more plastic deformation before fracture occurs, making the steel more ductile and less prone to brittle failure.
Brittleness:
Increasing the ferrite phase in steel can reduce its brittleness. Ferrite is a relatively soft and ductile phase, and its presence can prevent the formation of harder and more brittle phases, such as martensite or bainite. This leads to a more ductile steel that is less prone to sudden and catastrophic failure.
Conclusion:
In summary, increasing the ferrite phase in steel can increase its strength, improve ductility, and reduce brittleness. However, it generally decreases the hardness of the steel. These changes in mechanical properties are primarily due to the absence of hard and brittle phases, such as carbides or martensite, in the ferrite phase.
Diamagnetic materials- a)are nonmagnetic
- b)cannot be magnetized
- c)are magnetized in direction opposite to that of applied field
- d)can be magnetized in one direction only
Correct answer is option 'D'. Can you explain this answer?
Diamagnetic materials
a)
are nonmagnetic
b)
cannot be magnetized
c)
are magnetized in direction opposite to that of applied field
d)
can be magnetized in one direction only

|
Shail Rane answered |
Gibbs phase rule is given as
P + F = C + 2
Where P = Number of phase
F = Number of degree of freedom
C = Number of components
P + F = C + 2
Where P = Number of phase
F = Number of degree of freedom
C = Number of components
A material of unknown composition at atmospheric pressure (arbitrarily chosen) exhibit four phase at 987 K, what is the minimum number of components in the system?- a)2
- b)0
- c)3
- d)4
Correct answer is option 'C'. Can you explain this answer?
A material of unknown composition at atmospheric pressure (arbitrarily chosen) exhibit four phase at 987 K, what is the minimum number of components in the system?
a)
2
b)
0
c)
3
d)
4

|
Niharika Yadav answered |
To determine the minimum number of components in a system, we need to consider the phases present at a given temperature and pressure. In this case, the material exhibits four phases at a temperature of 987 K and atmospheric pressure.
The phases of a material refer to the different states it can exist in, such as solid, liquid, and gas. Each phase represents a different arrangement of the atoms or molecules in the material.
In the given scenario, we have four phases at 987 K. This means that there are four distinct arrangements of atoms or molecules in the material at this temperature.
To understand the minimum number of components, we need to consider the concept of phases and components in thermodynamics.
- Phases: A phase is a homogeneous portion of a material that is physically distinct and separated from other portions by definite boundaries. Each phase has its own set of physical properties, such as density, viscosity, and compressibility.
- Components: Components are the chemically independent constituents of a system. They are the minimum number of chemically independent constituents required to specify the composition of each phase.
Considering the given information, we have four phases at 987 K. The minimum number of components required to specify the composition of each phase is three (option C).
This means that there are three chemically independent constituents present in the system, which combine to form the four different phases observed at 987 K. The specific composition of each phase is determined by the combination and proportion of these three components.
It's important to note that without further information about the nature of the material and its phases, it is impossible to determine the exact composition of the components or their individual properties. The answer is based solely on the given information that there are four phases at 987 K.
The phases of a material refer to the different states it can exist in, such as solid, liquid, and gas. Each phase represents a different arrangement of the atoms or molecules in the material.
In the given scenario, we have four phases at 987 K. This means that there are four distinct arrangements of atoms or molecules in the material at this temperature.
To understand the minimum number of components, we need to consider the concept of phases and components in thermodynamics.
- Phases: A phase is a homogeneous portion of a material that is physically distinct and separated from other portions by definite boundaries. Each phase has its own set of physical properties, such as density, viscosity, and compressibility.
- Components: Components are the chemically independent constituents of a system. They are the minimum number of chemically independent constituents required to specify the composition of each phase.
Considering the given information, we have four phases at 987 K. The minimum number of components required to specify the composition of each phase is three (option C).
This means that there are three chemically independent constituents present in the system, which combine to form the four different phases observed at 987 K. The specific composition of each phase is determined by the combination and proportion of these three components.
It's important to note that without further information about the nature of the material and its phases, it is impossible to determine the exact composition of the components or their individual properties. The answer is based solely on the given information that there are four phases at 987 K.
Cementite consists of- a)13% Cementite, 87% Ferrite
- b)13% Carbon, 87% Ferrite
- c)13% Ferrite, 87% Cementite
- d)6,67% Carbon, 93.33% Iron
Correct answer is option 'C'. Can you explain this answer?
Cementite consists of
a)
13% Cementite, 87% Ferrite
b)
13% Carbon, 87% Ferrite
c)
13% Ferrite, 87% Cementite
d)
6,67% Carbon, 93.33% Iron
|
|
Disha Nambiar answered |
Austenite is a solid solution of carbon in γ iron which is not stable at room temperature.
Hyper eutectoid steels have carbon content- a)equal to 0.83%
- b)more than 0.83% and upto 2%
- c)less than 0.83%
- d)more than 2%
Correct answer is option 'B'. Can you explain this answer?
Hyper eutectoid steels have carbon content
a)
equal to 0.83%
b)
more than 0.83% and upto 2%
c)
less than 0.83%
d)
more than 2%

|
Bijoy Mehra answered |
Hyper eutectoid steels are a type of steel that contain more than 0.83% carbon and up to 2% carbon. Let's understand why the correct answer is option 'B' in detail.
Definition of Hyper Eutectoid Steels
- Eutectoid steels are a type of steel that contain exactly 0.83% carbon, which is the eutectoid composition.
- Hyper eutectoid steels, on the other hand, have a carbon content greater than the eutectoid composition, meaning they contain more than 0.83% carbon.
Explanation of the Correct Answer
The correct answer is option 'B', which states that hyper eutectoid steels have carbon content more than 0.83% and up to 2%. This means that the carbon content in hyper eutectoid steels ranges from more than 0.83% to a maximum of 2%.
Importance of Carbon Content in Steels
- The carbon content in steels plays a crucial role in determining their mechanical properties and behavior.
- Low carbon steels (carbon content less than 0.25%) have good ductility and are easily weldable, but they have lower strength and hardness.
- Medium carbon steels (carbon content between 0.25% and 0.60%) have higher strength and hardness, but they are less ductile and less weldable.
- High carbon steels (carbon content greater than 0.60%) have the highest strength and hardness, but they are brittle and difficult to weld.
Properties of Hyper Eutectoid Steels
Hyper eutectoid steels, with a carbon content greater than 0.83% and up to 2%, have the following properties:
- High hardness: The increased carbon content allows for greater hardness in the steel, making it suitable for applications that require wear resistance, such as tools and blades.
- Reduced ductility: As the carbon content increases, the ductility of the steel decreases. Hyper eutectoid steels may exhibit lower ductility compared to lower carbon steels, which can make them more prone to cracking or breaking under certain conditions.
- Increased brittleness: Hyper eutectoid steels with carbon content closer to the upper limit of 2% can become increasingly brittle, reducing their toughness and impact resistance.
Applications of Hyper Eutectoid Steels
- Hyper eutectoid steels are commonly used in applications that require high hardness and wear resistance, such as cutting tools, springs, and high-strength wires.
- These steels are also suitable for applications where the material needs to retain its shape under high stress or in harsh environments.
In conclusion, hyper eutectoid steels have a carbon content greater than 0.83% and up to 2%, making them harder and less ductile compared to lower carbon steels. They find applications in industries where high hardness and wear resistance are required.
Definition of Hyper Eutectoid Steels
- Eutectoid steels are a type of steel that contain exactly 0.83% carbon, which is the eutectoid composition.
- Hyper eutectoid steels, on the other hand, have a carbon content greater than the eutectoid composition, meaning they contain more than 0.83% carbon.
Explanation of the Correct Answer
The correct answer is option 'B', which states that hyper eutectoid steels have carbon content more than 0.83% and up to 2%. This means that the carbon content in hyper eutectoid steels ranges from more than 0.83% to a maximum of 2%.
Importance of Carbon Content in Steels
- The carbon content in steels plays a crucial role in determining their mechanical properties and behavior.
- Low carbon steels (carbon content less than 0.25%) have good ductility and are easily weldable, but they have lower strength and hardness.
- Medium carbon steels (carbon content between 0.25% and 0.60%) have higher strength and hardness, but they are less ductile and less weldable.
- High carbon steels (carbon content greater than 0.60%) have the highest strength and hardness, but they are brittle and difficult to weld.
Properties of Hyper Eutectoid Steels
Hyper eutectoid steels, with a carbon content greater than 0.83% and up to 2%, have the following properties:
- High hardness: The increased carbon content allows for greater hardness in the steel, making it suitable for applications that require wear resistance, such as tools and blades.
- Reduced ductility: As the carbon content increases, the ductility of the steel decreases. Hyper eutectoid steels may exhibit lower ductility compared to lower carbon steels, which can make them more prone to cracking or breaking under certain conditions.
- Increased brittleness: Hyper eutectoid steels with carbon content closer to the upper limit of 2% can become increasingly brittle, reducing their toughness and impact resistance.
Applications of Hyper Eutectoid Steels
- Hyper eutectoid steels are commonly used in applications that require high hardness and wear resistance, such as cutting tools, springs, and high-strength wires.
- These steels are also suitable for applications where the material needs to retain its shape under high stress or in harsh environments.
In conclusion, hyper eutectoid steels have a carbon content greater than 0.83% and up to 2%, making them harder and less ductile compared to lower carbon steels. They find applications in industries where high hardness and wear resistance are required.
The structure of austenite is- a)body-centered cubic
- b)face-centered cubic
- c)hexagonal close-packed
- d)body-centered tetragonal
Correct answer is option 'B'. Can you explain this answer?
The structure of austenite is
a)
body-centered cubic
b)
face-centered cubic
c)
hexagonal close-packed
d)
body-centered tetragonal

|
Anuj Chakraborty answered |
Structure of Austenite
Austenite is a solid solution of carbon and iron that exists in the face-centered cubic (FCC) crystal structure. This structure is characterized by atoms arranged in a cubic close-packed manner, with one atom at each corner of the cube and one atom at the center of each face.
Face-Centered Cubic (FCC) Structure
In the face-centered cubic structure, the atoms are packed closely together, maximizing the density of the material. This structure provides good ductility and formability to the material, making it suitable for applications where deformation is required.
Characteristics of FCC Structure
- Atoms are arranged in a cubic pattern with one atom at each corner and one atom at the center of each face.
- High packing efficiency, resulting in high density and good mechanical properties.
- Good ductility and formability due to the close-packed nature of the structure.
- FCC metals tend to have higher melting points compared to body-centered cubic (BCC) metals.
Importance of Austenite Structure
The FCC structure of austenite allows it to exhibit properties such as high ductility, good formability, and resistance to deformation. These characteristics make austenite suitable for a wide range of applications in industries such as automotive, aerospace, and construction.
In conclusion, austenite has a face-centered cubic structure, which contributes to its desirable mechanical properties and versatility in various engineering applications.
Austenite is a solid solution of carbon and iron that exists in the face-centered cubic (FCC) crystal structure. This structure is characterized by atoms arranged in a cubic close-packed manner, with one atom at each corner of the cube and one atom at the center of each face.
Face-Centered Cubic (FCC) Structure
In the face-centered cubic structure, the atoms are packed closely together, maximizing the density of the material. This structure provides good ductility and formability to the material, making it suitable for applications where deformation is required.
Characteristics of FCC Structure
- Atoms are arranged in a cubic pattern with one atom at each corner and one atom at the center of each face.
- High packing efficiency, resulting in high density and good mechanical properties.
- Good ductility and formability due to the close-packed nature of the structure.
- FCC metals tend to have higher melting points compared to body-centered cubic (BCC) metals.
Importance of Austenite Structure
The FCC structure of austenite allows it to exhibit properties such as high ductility, good formability, and resistance to deformation. These characteristics make austenite suitable for a wide range of applications in industries such as automotive, aerospace, and construction.
In conclusion, austenite has a face-centered cubic structure, which contributes to its desirable mechanical properties and versatility in various engineering applications.
A material is said to be allotropic. If it has- a)fixed structure at all temperatures .
- b)atoms distributed in random pattern
- c)different crystal structures at different temperature
- d)any of the above
Correct answer is option 'C'. Can you explain this answer?
A material is said to be allotropic. If it has
a)
fixed structure at all temperatures .
b)
atoms distributed in random pattern
c)
different crystal structures at different temperature
d)
any of the above

|
Aditya Jain answered |
Understanding Allotropy
Allotropy is a phenomenon where a material can exist in different structural forms. The key aspect of allotropy is that these different forms are stable under specific conditions, such as temperature and pressure.
Why Option C is Correct
- Allotropic materials exhibit different crystal structures at different temperatures.
- For example, carbon is known to exist in various forms, such as diamond and graphite, which have distinct arrangements of atoms.
- At elevated temperatures, certain materials may transition into another allotrope, showcasing different physical and chemical properties.
Why Other Options are Incorrect
- Option A: Fixed Structure at All Temperatures
- This is not true for allotropic materials. They can change their structure with varying conditions, particularly temperature.
- Option B: Atoms Distributed in Random Pattern
- This describes amorphous materials rather than allotropy. While some allotropes might exhibit some randomness, allotropy specifically refers to distinct crystalline structures.
Conclusion
In summary, allotropy is defined by the ability of a material to have different crystal structures at various temperatures. Understanding this concept is crucial in materials science and engineering, as it affects the properties and applications of materials in different environments.
Allotropy is a phenomenon where a material can exist in different structural forms. The key aspect of allotropy is that these different forms are stable under specific conditions, such as temperature and pressure.
Why Option C is Correct
- Allotropic materials exhibit different crystal structures at different temperatures.
- For example, carbon is known to exist in various forms, such as diamond and graphite, which have distinct arrangements of atoms.
- At elevated temperatures, certain materials may transition into another allotrope, showcasing different physical and chemical properties.
Why Other Options are Incorrect
- Option A: Fixed Structure at All Temperatures
- This is not true for allotropic materials. They can change their structure with varying conditions, particularly temperature.
- Option B: Atoms Distributed in Random Pattern
- This describes amorphous materials rather than allotropy. While some allotropes might exhibit some randomness, allotropy specifically refers to distinct crystalline structures.
Conclusion
In summary, allotropy is defined by the ability of a material to have different crystal structures at various temperatures. Understanding this concept is crucial in materials science and engineering, as it affects the properties and applications of materials in different environments.
Nose of a C-curve represents- a)Shortest time required for specified fraction of transformation
- b)Longest time required for specified fraction of transformation
- c)Average time required for specified fraction of transformation
- d)No information regarding time required for specified fraction of transformation
Correct answer is option 'A'. Can you explain this answer?
Nose of a C-curve represents
a)
Shortest time required for specified fraction of transformation
b)
Longest time required for specified fraction of transformation
c)
Average time required for specified fraction of transformation
d)
No information regarding time required for specified fraction of transformation
|
|
Hiral Jain answered |
Explanation:
The nose of a C-curve represents the shortest time required for a specified fraction of transformation. This is an important concept in materials science and engineering, particularly in the study of phase transformations and heat treatment of metals.
C-curve is a graph that shows the variation of the fraction transformed with time during a heat treatment process. It is used to study the kinetics of phase transformations in materials. The curve has a characteristic shape, with a steep rise at the beginning, followed by a gradual slope and finally a plateau. The steep rise at the beginning of the curve is called the nose.
The nose of the C-curve represents the shortest time required for a specified fraction of transformation to occur. This is because the nose is the point where the transformation rate is at its maximum. As the transformation proceeds, the rate decreases, and the curve becomes less steep.
The nose is an important parameter in the study of phase transformations because it provides information about the kinetics of the process. By measuring the position of the nose on the C-curve, it is possible to determine the activation energy of the transformation and other kinetic parameters. This information is useful in designing heat treatment processes for specific applications.
In summary, the nose of a C-curve represents the shortest time required for a specified fraction of transformation to occur. It is an important parameter in the study of phase transformations and provides valuable information about the kinetics of the process.
The nose of a C-curve represents the shortest time required for a specified fraction of transformation. This is an important concept in materials science and engineering, particularly in the study of phase transformations and heat treatment of metals.
C-curve is a graph that shows the variation of the fraction transformed with time during a heat treatment process. It is used to study the kinetics of phase transformations in materials. The curve has a characteristic shape, with a steep rise at the beginning, followed by a gradual slope and finally a plateau. The steep rise at the beginning of the curve is called the nose.
The nose of the C-curve represents the shortest time required for a specified fraction of transformation to occur. This is because the nose is the point where the transformation rate is at its maximum. As the transformation proceeds, the rate decreases, and the curve becomes less steep.
The nose is an important parameter in the study of phase transformations because it provides information about the kinetics of the process. By measuring the position of the nose on the C-curve, it is possible to determine the activation energy of the transformation and other kinetic parameters. This information is useful in designing heat treatment processes for specific applications.
In summary, the nose of a C-curve represents the shortest time required for a specified fraction of transformation to occur. It is an important parameter in the study of phase transformations and provides valuable information about the kinetics of the process.
The maximum number of co-existing phases in a C- system is- a)C-1
- b)C+2
- c)P(C -1)
- d)C-P+2
Correct answer is option 'B'. Can you explain this answer?
The maximum number of co-existing phases in a C- system is
a)
C-1
b)
C+2
c)
P(C -1)
d)
C-P+2

|
Nilanjan Rane answered |
The maximum number of co-existing phases in a C-system is C-1.
Explanation:
A C-system refers to a system that contains multiple components. Each component can exist in different phases, such as solid, liquid, or gas. The maximum number of co-existing phases in a C-system can be determined using the Gibbs phase rule.
The Gibbs phase rule states that for a system with C components and P phases, the number of degrees of freedom (F) is given by:
F = C - P + 2
In this equation, F represents the number of independent variables that can be varied without changing the number of phases.
To determine the maximum number of co-existing phases, we need to consider the condition where F is equal to zero. This means that there are no independent variables and the system is at equilibrium.
Setting F = 0 in the Gibbs phase rule equation, we have:
0 = C - P + 2
Rearranging the equation, we get:
P = C + 2
Therefore, the maximum number of co-existing phases in a C-system is C + 2.
However, we need to subtract 1 from the result because one phase is always a reference phase, usually the standard state of the pure component. This reference phase is necessary to define the properties and behavior of the other phases.
Hence, the correct answer is option (b) C-1, which represents the maximum number of co-existing phases in a C-system.
Explanation:
A C-system refers to a system that contains multiple components. Each component can exist in different phases, such as solid, liquid, or gas. The maximum number of co-existing phases in a C-system can be determined using the Gibbs phase rule.
The Gibbs phase rule states that for a system with C components and P phases, the number of degrees of freedom (F) is given by:
F = C - P + 2
In this equation, F represents the number of independent variables that can be varied without changing the number of phases.
To determine the maximum number of co-existing phases, we need to consider the condition where F is equal to zero. This means that there are no independent variables and the system is at equilibrium.
Setting F = 0 in the Gibbs phase rule equation, we have:
0 = C - P + 2
Rearranging the equation, we get:
P = C + 2
Therefore, the maximum number of co-existing phases in a C-system is C + 2.
However, we need to subtract 1 from the result because one phase is always a reference phase, usually the standard state of the pure component. This reference phase is necessary to define the properties and behavior of the other phases.
Hence, the correct answer is option (b) C-1, which represents the maximum number of co-existing phases in a C-system.
Horizontal arrest in a cooling curve represents:- a)Continuous cooling
- b)Invariant reaction
- c)Discrete cooling
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
Horizontal arrest in a cooling curve represents:
a)
Continuous cooling
b)
Invariant reaction
c)
Discrete cooling
d)
None of these
|
|
Arnav Menon answered |
Horizontal arrest in a cooling curve represents an invariant reaction.
Explanation:
When a material undergoes a phase change, such as solidification or crystallization, its cooling curve shows a characteristic pattern. This cooling curve represents the relationship between temperature and time during the cooling process.
Cooling Curve:
A cooling curve is a graphical representation of the temperature changes that occur as a material cools from a higher temperature to a lower temperature. It typically consists of a downward-sloping line that represents the cooling process.
Horizontal Arrest:
Horizontal arrest refers to a specific region in the cooling curve where the temperature remains constant for a period of time. This region is characterized by a flat, horizontal line on the cooling curve.
Invariant Reaction:
An invariant reaction is a phase change that occurs at a constant temperature and pressure. It is a reaction in which the composition and properties of the material do not change during the process. In other words, the material undergoes a phase change without any further cooling or heating.
Horizontal Arrest Represents Invariant Reaction:
The presence of a horizontal arrest in a cooling curve indicates that an invariant reaction is occurring. During this phase, the material is undergoing a phase change, such as solidification or crystallization, which occurs at a constant temperature.
Characteristics of Horizontal Arrest:
1. Constant Temperature: The temperature remains constant during the horizontal arrest phase.
2. No Heat Exchange: There is no heat exchange between the material and its surroundings during the horizontal arrest phase.
3. Phase Change: The material is undergoing a phase change, such as the formation of solid crystals or the transformation of a liquid to a solid.
4. Stable Composition: The composition and properties of the material remain constant during the horizontal arrest phase.
Conclusion:
In summary, horizontal arrest in a cooling curve represents an invariant reaction, which is a phase change occurring at a constant temperature. It is characterized by a flat, horizontal line on the cooling curve, indicating a stable composition and no heat exchange during the phase change.
Explanation:
When a material undergoes a phase change, such as solidification or crystallization, its cooling curve shows a characteristic pattern. This cooling curve represents the relationship between temperature and time during the cooling process.
Cooling Curve:
A cooling curve is a graphical representation of the temperature changes that occur as a material cools from a higher temperature to a lower temperature. It typically consists of a downward-sloping line that represents the cooling process.
Horizontal Arrest:
Horizontal arrest refers to a specific region in the cooling curve where the temperature remains constant for a period of time. This region is characterized by a flat, horizontal line on the cooling curve.
Invariant Reaction:
An invariant reaction is a phase change that occurs at a constant temperature and pressure. It is a reaction in which the composition and properties of the material do not change during the process. In other words, the material undergoes a phase change without any further cooling or heating.
Horizontal Arrest Represents Invariant Reaction:
The presence of a horizontal arrest in a cooling curve indicates that an invariant reaction is occurring. During this phase, the material is undergoing a phase change, such as solidification or crystallization, which occurs at a constant temperature.
Characteristics of Horizontal Arrest:
1. Constant Temperature: The temperature remains constant during the horizontal arrest phase.
2. No Heat Exchange: There is no heat exchange between the material and its surroundings during the horizontal arrest phase.
3. Phase Change: The material is undergoing a phase change, such as the formation of solid crystals or the transformation of a liquid to a solid.
4. Stable Composition: The composition and properties of the material remain constant during the horizontal arrest phase.
Conclusion:
In summary, horizontal arrest in a cooling curve represents an invariant reaction, which is a phase change occurring at a constant temperature. It is characterized by a flat, horizontal line on the cooling curve, indicating a stable composition and no heat exchange during the phase change.
Hardness of steel depends- a)Amount of fabrication
- b)The shape and distribution of the carbides in iron
- c)Method of fabrication
- d)Contents of alloying elements
Correct answer is option 'B'. Can you explain this answer?
Hardness of steel depends
a)
Amount of fabrication
b)
The shape and distribution of the carbides in iron
c)
Method of fabrication
d)
Contents of alloying elements

|
Raghavendra Dasgupta answered |
Increasing the carbon content in steel alloys causes the material to become harder. This is because the carbon sits in the interstitial sites of the lattice structure and hinders the movement of dislocation lines. It also increases the strength of the material but it decreases the ductility, carbide is hard phase,
Martensite is the super saturated solution of carbon in- a)Iron
- b)Steel
- c)Alpha-Iron
- d)Beta Iron
Correct answer is option 'A'. Can you explain this answer?
Martensite is the super saturated solution of carbon in
a)
Iron
b)
Steel
c)
Alpha-Iron
d)
Beta Iron
|
|
Soumya Basak answered |
**Martensite:**
Martensite is a type of microstructure that forms in certain steels when they are rapidly cooled from a high temperature. It is characterized by a needle-like or plate-like structure and is extremely hard and brittle. Martensite is formed due to a diffusionless transformation, meaning that it occurs without the movement of atoms.
**Super Saturated Solution:**
A super saturated solution is a solution that contains more solute than it should be able to hold at a given temperature. In other words, it is a solution that is unstable and contains an excess amount of solute.
**Martensite as a Super Saturated Solution of Carbon:**
In the case of martensite, it is considered a super saturated solution of carbon in iron. This means that the martensite structure contains more carbon atoms than can normally be accommodated in the iron lattice.
**Explanation:**
When steel is heated to high temperatures, the carbon atoms dissolve into the iron lattice, forming a solid solution. This is known as austenite, which has a face-centered cubic (FCC) crystal structure. When the steel is rapidly cooled, the carbon atoms are trapped in the iron lattice, leading to a supersaturated solution.
The rapid cooling process prevents the carbon atoms from diffusing out of the lattice and forming cementite, which is a compound of iron and carbon with a distinct crystal structure. As a result, the excess carbon remains trapped in the iron lattice, resulting in a super saturated solution known as martensite.
**Significance of Martensite:**
Martensite is significant because it imparts hardness and strength to steels. It is harder and stronger than the original austenite structure. However, it is also extremely brittle, which can limit its applications. Therefore, it is often desirable to temper martensite to reduce its brittleness and improve its toughness.
**Conclusion:**
In conclusion, martensite is a super saturated solution of carbon in iron. It forms when steel is rapidly cooled, trapping excess carbon atoms in the iron lattice. Martensite is known for its hardness and strength but is also brittle. It is an important microstructure in the field of metallurgy, especially in the development of high-strength steels.
Martensite is a type of microstructure that forms in certain steels when they are rapidly cooled from a high temperature. It is characterized by a needle-like or plate-like structure and is extremely hard and brittle. Martensite is formed due to a diffusionless transformation, meaning that it occurs without the movement of atoms.
**Super Saturated Solution:**
A super saturated solution is a solution that contains more solute than it should be able to hold at a given temperature. In other words, it is a solution that is unstable and contains an excess amount of solute.
**Martensite as a Super Saturated Solution of Carbon:**
In the case of martensite, it is considered a super saturated solution of carbon in iron. This means that the martensite structure contains more carbon atoms than can normally be accommodated in the iron lattice.
**Explanation:**
When steel is heated to high temperatures, the carbon atoms dissolve into the iron lattice, forming a solid solution. This is known as austenite, which has a face-centered cubic (FCC) crystal structure. When the steel is rapidly cooled, the carbon atoms are trapped in the iron lattice, leading to a supersaturated solution.
The rapid cooling process prevents the carbon atoms from diffusing out of the lattice and forming cementite, which is a compound of iron and carbon with a distinct crystal structure. As a result, the excess carbon remains trapped in the iron lattice, resulting in a super saturated solution known as martensite.
**Significance of Martensite:**
Martensite is significant because it imparts hardness and strength to steels. It is harder and stronger than the original austenite structure. However, it is also extremely brittle, which can limit its applications. Therefore, it is often desirable to temper martensite to reduce its brittleness and improve its toughness.
**Conclusion:**
In conclusion, martensite is a super saturated solution of carbon in iron. It forms when steel is rapidly cooled, trapping excess carbon atoms in the iron lattice. Martensite is known for its hardness and strength but is also brittle. It is an important microstructure in the field of metallurgy, especially in the development of high-strength steels.
Which of the following is not a typical site for nucleation during solid stage transformation?- a)Container wall
- b)Grain boundaries
- c)Stacking faults
- d)Dislocations
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not a typical site for nucleation during solid stage transformation?
a)
Container wall
b)
Grain boundaries
c)
Stacking faults
d)
Dislocations

|
Tanvi Sarkar answered |
Nucleation during solid stage transformation
During solid stage transformation, nucleation is the process by which new solid phases are formed. Nucleation occurs at specific sites within the material, providing a starting point for the growth of the new phase. These nucleation sites play a crucial role in determining the final microstructure and properties of the material.
Typical sites for nucleation
The typical sites for nucleation during solid stage transformation include:
- Grain boundaries: Grain boundaries are the interfaces between adjacent grains in a polycrystalline material. They often serve as nucleation sites due to their high energy and the presence of defects, such as dislocations and stacking faults.
- Stacking faults: Stacking faults are planar defects that occur when there is a deviation from the regular stacking sequence of atoms in a crystal lattice. These faults can act as nucleation sites for the formation of new phases.
- Dislocations: Dislocations are line defects in the crystal lattice that result from the presence of extra or missing atoms. They can provide sites for the nucleation of new phases during solid stage transformation.
Atypical site for nucleation
The site that is not typically associated with nucleation during solid stage transformation is the container wall. The container wall refers to the surface of the container in which the material is being processed or transformed. While the container wall can influence the overall transformation process, it is not a typical site for nucleation.
Explanation
Nucleation occurs within the bulk of the material rather than at the container wall. This is because nucleation requires certain conditions, such as the presence of defects or high-energy interfaces, which are typically found within the material itself. The container wall, on the other hand, is a relatively low-energy surface and does not provide the necessary conditions for nucleation to occur.
Furthermore, nucleation at the container wall would result in the formation of new phases only on the surface of the material, rather than throughout the bulk. This would lead to a non-uniform microstructure and potentially undesirable properties.
In summary, the container wall is not a typical site for nucleation during solid stage transformation. Nucleation more commonly occurs at grain boundaries, stacking faults, and dislocations within the material itself.
During solid stage transformation, nucleation is the process by which new solid phases are formed. Nucleation occurs at specific sites within the material, providing a starting point for the growth of the new phase. These nucleation sites play a crucial role in determining the final microstructure and properties of the material.
Typical sites for nucleation
The typical sites for nucleation during solid stage transformation include:
- Grain boundaries: Grain boundaries are the interfaces between adjacent grains in a polycrystalline material. They often serve as nucleation sites due to their high energy and the presence of defects, such as dislocations and stacking faults.
- Stacking faults: Stacking faults are planar defects that occur when there is a deviation from the regular stacking sequence of atoms in a crystal lattice. These faults can act as nucleation sites for the formation of new phases.
- Dislocations: Dislocations are line defects in the crystal lattice that result from the presence of extra or missing atoms. They can provide sites for the nucleation of new phases during solid stage transformation.
Atypical site for nucleation
The site that is not typically associated with nucleation during solid stage transformation is the container wall. The container wall refers to the surface of the container in which the material is being processed or transformed. While the container wall can influence the overall transformation process, it is not a typical site for nucleation.
Explanation
Nucleation occurs within the bulk of the material rather than at the container wall. This is because nucleation requires certain conditions, such as the presence of defects or high-energy interfaces, which are typically found within the material itself. The container wall, on the other hand, is a relatively low-energy surface and does not provide the necessary conditions for nucleation to occur.
Furthermore, nucleation at the container wall would result in the formation of new phases only on the surface of the material, rather than throughout the bulk. This would lead to a non-uniform microstructure and potentially undesirable properties.
In summary, the container wall is not a typical site for nucleation during solid stage transformation. Nucleation more commonly occurs at grain boundaries, stacking faults, and dislocations within the material itself.
In iron-carbide diagram, pearlite is- a)Eutectoid mixture of cementite and ferrite
- b)Eutectoid mixture of austenite and ferrite
- c)Eutectic mixture of austenite and cementite
- d)Eutectic mixture of austenite and ferrite
Correct answer is option 'A'. Can you explain this answer?
In iron-carbide diagram, pearlite is
a)
Eutectoid mixture of cementite and ferrite
b)
Eutectoid mixture of austenite and ferrite
c)
Eutectic mixture of austenite and cementite
d)
Eutectic mixture of austenite and ferrite

|
Siddharth Bajaj answered |
Understanding the Iron-Carbide Diagram
The iron-carbide diagram (also known as the Fe-C phase diagram) illustrates the phase transformations in iron-carbon alloys. The diagram is crucial for understanding the microstructural constituents of steel and cast iron.
What is Pearlite?
- Pearlite is a significant microstructural component formed during the cooling of austenite (the high-temperature phase of steel).
- It is defined as a eutectoid mixture, meaning it forms at a specific composition and temperature.
Eutectoid Composition
- The eutectoid point in the iron-carbon diagram occurs at approximately 0.76% carbon content and a temperature of about 727°C.
- At this point, when austenite transforms upon cooling, it changes into a mixture of cementite (Fe3C) and ferrite (α-iron).
Why Option A is Correct?
- Option A states that pearlite is a "eutectoid mixture of cementite and ferrite." This is accurate because:
- Pearlite consists of alternating layers of ferrite and cementite, giving it a lamellar structure.
- The formation of pearlite occurs when austenite undergoes a eutectoid transformation during cooling, resulting in this specific mixture.
Other Options Explained
- Option B: Incorrect, as pearlite is not formed from austenite and ferrite directly.
- Option C: Incorrect, as it suggests a eutectic mixture which is distinct from eutectoid.
- Option D: Incorrect, as it mischaracterizes the components involved in pearlite formation.
In summary, pearlite is indeed a eutectoid mixture of cementite and ferrite, and understanding its formation is crucial for mechanical engineering applications involving steel and its properties.
The iron-carbide diagram (also known as the Fe-C phase diagram) illustrates the phase transformations in iron-carbon alloys. The diagram is crucial for understanding the microstructural constituents of steel and cast iron.
What is Pearlite?
- Pearlite is a significant microstructural component formed during the cooling of austenite (the high-temperature phase of steel).
- It is defined as a eutectoid mixture, meaning it forms at a specific composition and temperature.
Eutectoid Composition
- The eutectoid point in the iron-carbon diagram occurs at approximately 0.76% carbon content and a temperature of about 727°C.
- At this point, when austenite transforms upon cooling, it changes into a mixture of cementite (Fe3C) and ferrite (α-iron).
Why Option A is Correct?
- Option A states that pearlite is a "eutectoid mixture of cementite and ferrite." This is accurate because:
- Pearlite consists of alternating layers of ferrite and cementite, giving it a lamellar structure.
- The formation of pearlite occurs when austenite undergoes a eutectoid transformation during cooling, resulting in this specific mixture.
Other Options Explained
- Option B: Incorrect, as pearlite is not formed from austenite and ferrite directly.
- Option C: Incorrect, as it suggests a eutectic mixture which is distinct from eutectoid.
- Option D: Incorrect, as it mischaracterizes the components involved in pearlite formation.
In summary, pearlite is indeed a eutectoid mixture of cementite and ferrite, and understanding its formation is crucial for mechanical engineering applications involving steel and its properties.
The reaction that on heating one solid phase yields another solid phase together with one liquid phase is termed- a)peritectic
- b)peritectoid
- c)eutectic
- d)eutectoid
Correct answer is option 'A'. Can you explain this answer?
The reaction that on heating one solid phase yields another solid phase together with one liquid phase is termed
a)
peritectic
b)
peritectoid
c)
eutectic
d)
eutectoid

|
Ankit Mukherjee answered |
Peritectic Reaction
A peritectic reaction refers to a type of solid-state phase transformation that occurs when one solid phase reacts with a liquid phase to form another solid phase. This reaction is typically initiated by heating the system.
Explanation:
- The peritectic reaction is characterized by the formation of a liquid phase at a specific temperature and composition, known as the peritectic temperature and peritectic composition, respectively.
- At temperatures below the peritectic temperature, the system consists of only the initial solid phase.
- As the temperature increases, the liquid phase begins to form at the peritectic composition, causing a coexistence of the initial solid phase and the liquid phase.
- The liquid phase then reacts with the initial solid phase to form a new solid phase, resulting in the disappearance of the liquid phase.
- The reaction is complete when only the new solid phase remains.
Peritectic vs. Eutectic and Eutectoid Reactions:
- While the peritectic reaction involves the formation of a liquid phase, the eutectic and eutectoid reactions involve the formation of a different solid phase.
- In a eutectic reaction, a liquid phase reacts with two or more solid phases to form a new solid phase.
- In a eutectoid reaction, one solid phase decomposes into two new solid phases without the presence of a liquid phase.
Examples of Peritectic Reactions:
- One example of a peritectic reaction is the formation of cementite (Fe3C) in steel.
- At temperatures below the peritectic temperature, the system consists of ferrite (α-Fe) as the initial solid phase.
- As the temperature increases, a liquid phase forms at the peritectic composition.
- The liquid phase reacts with the ferrite to form cementite (Fe3C), resulting in the disappearance of the liquid phase.
- Another example is the formation of silicon carbide (SiC) in the reaction between silicon (Si) and carbon (C).
- At temperatures below the peritectic temperature, the system consists of silicon as the initial solid phase.
- As the temperature increases, a liquid phase forms at the peritectic composition.
- The liquid phase reacts with silicon to form silicon carbide, resulting in the disappearance of the liquid phase.
In conclusion, a peritectic reaction refers to a phase transformation in which one solid phase reacts with a liquid phase to form another solid phase. This reaction is characterized by the formation and subsequent disappearance of the liquid phase at a specific temperature and composition.
A peritectic reaction refers to a type of solid-state phase transformation that occurs when one solid phase reacts with a liquid phase to form another solid phase. This reaction is typically initiated by heating the system.
Explanation:
- The peritectic reaction is characterized by the formation of a liquid phase at a specific temperature and composition, known as the peritectic temperature and peritectic composition, respectively.
- At temperatures below the peritectic temperature, the system consists of only the initial solid phase.
- As the temperature increases, the liquid phase begins to form at the peritectic composition, causing a coexistence of the initial solid phase and the liquid phase.
- The liquid phase then reacts with the initial solid phase to form a new solid phase, resulting in the disappearance of the liquid phase.
- The reaction is complete when only the new solid phase remains.
Peritectic vs. Eutectic and Eutectoid Reactions:
- While the peritectic reaction involves the formation of a liquid phase, the eutectic and eutectoid reactions involve the formation of a different solid phase.
- In a eutectic reaction, a liquid phase reacts with two or more solid phases to form a new solid phase.
- In a eutectoid reaction, one solid phase decomposes into two new solid phases without the presence of a liquid phase.
Examples of Peritectic Reactions:
- One example of a peritectic reaction is the formation of cementite (Fe3C) in steel.
- At temperatures below the peritectic temperature, the system consists of ferrite (α-Fe) as the initial solid phase.
- As the temperature increases, a liquid phase forms at the peritectic composition.
- The liquid phase reacts with the ferrite to form cementite (Fe3C), resulting in the disappearance of the liquid phase.
- Another example is the formation of silicon carbide (SiC) in the reaction between silicon (Si) and carbon (C).
- At temperatures below the peritectic temperature, the system consists of silicon as the initial solid phase.
- As the temperature increases, a liquid phase forms at the peritectic composition.
- The liquid phase reacts with silicon to form silicon carbide, resulting in the disappearance of the liquid phase.
In conclusion, a peritectic reaction refers to a phase transformation in which one solid phase reacts with a liquid phase to form another solid phase. This reaction is characterized by the formation and subsequent disappearance of the liquid phase at a specific temperature and composition.
Which one of the following is the correct statements?- a)Phase consisting of ferrite and cementite at room temperature
- b)Mechanical mixture of ferrite and cementite at room temperature
- c)Eutectic mixture of ferrite and cementite at room temperature
- d)All the above the three are correct
Correct answer is option 'B'. Can you explain this answer?
Which one of the following is the correct statements?
a)
Phase consisting of ferrite and cementite at room temperature
b)
Mechanical mixture of ferrite and cementite at room temperature
c)
Eutectic mixture of ferrite and cementite at room temperature
d)
All the above the three are correct
|
|
Nandita Chakraborty answered |
The mechanical mixture of ferrite and cementite at room temperature is called pearlite, due to its pearly appearance under the optical microscope.
A given steel test specimen is studied under metallurgical microscope. Magnification used is 100 X. In that different phases are observed. One of them is Fe3C.- a)ferrite
- b)cementite
- c)austenite
- d)martensite
Correct answer is option 'B'. Can you explain this answer?
A given steel test specimen is studied under metallurgical microscope. Magnification used is 100 X. In that different phases are observed. One of them is Fe3C.
a)
ferrite
b)
cementite
c)
austenite
d)
martensite
|
|
Dipika Bose answered |
Cementite has Fe3C composition, inter metallic compound.
Overall transformation rate changes with temperature as follows:- a)Monotonically decreases with temperature
- b)First increases, then decreases
- c)Initially it is slow, and then picks up
- d)Monotonically increases with temperature
Correct answer is option 'B'. Can you explain this answer?
Overall transformation rate changes with temperature as follows:
a)
Monotonically decreases with temperature
b)
First increases, then decreases
c)
Initially it is slow, and then picks up
d)
Monotonically increases with temperature

|
Bhaskar Mukherjee answered |
Explanation:
Introduction:
When studying the transformation rate changes with temperature, it is important to understand the behavior of the process as the temperature increases or decreases.
First increases, then decreases:
- Initially, as the temperature increases, the transformation rate tends to increase due to the activation energy required for the process.
- However, after reaching a certain temperature threshold, the rate starts to decrease as the system may reach equilibrium or the reaction may become less favorable at higher temperatures.
Reasoning:
- At low temperatures, the kinetics of the transformation may be slow due to insufficient energy for the process to occur efficiently.
- As the temperature rises, more energy becomes available, leading to an increase in the transformation rate.
- Eventually, at higher temperatures, other factors such as thermodynamic constraints or the breakdown of reactants may cause the rate to decline.
Implications:
- Understanding this temperature-dependent behavior is crucial for optimizing processes and predicting outcomes in various fields such as materials science, chemical engineering, and biological systems.
- By studying the transformation rate changes with temperature, researchers can determine the optimal conditions for a specific process and improve efficiency.
Conclusion:
In conclusion, the transformation rate typically follows a pattern of first increasing with temperature and then decreasing after reaching a peak. This behavior is influenced by factors such as activation energy, thermodynamic constraints, and the stability of reactants. By recognizing this trend, scientists and engineers can make informed decisions when designing and optimizing processes.
In peritectoid reaction on cooling, we get one solid phase from- a)two liquid phases
- b)two solid phases
- c)one solid & one liquid phase
- d)none of these
Correct answer is option 'B'. Can you explain this answer?
In peritectoid reaction on cooling, we get one solid phase from
a)
two liquid phases
b)
two solid phases
c)
one solid & one liquid phase
d)
none of these

|
Rutuja Deshpande answered |
Phase and one liquid phased)one liquid phase and one gas phase
b) two solid phases
b) two solid phases
Weight % of carbon in mild steels is
- a)< 0.008
- b)0.008 - 0.3
- c)0.3 - 0.8
- d)0.8 - 2.11
Correct answer is option 'B'. Can you explain this answer?
Weight % of carbon in mild steels is
a)
< 0.008
b)
0.008 - 0.3
c)
0.3 - 0.8
d)
0.8 - 2.11

|
Anuj Verma answered |
0.05-0.25%
b) 0.25-0.45%
c) 0.45-0.60%
d) 0.60-0.80%
b) 0.25-0.45%
c) 0.45-0.60%
d) 0.60-0.80%
Which of the following is not a Hume-Ruthery condition?- a)Crystal structure of each element of solid solution must be the same.
- b)Size of atoms of each two elements must not differ by more than 15%.
- c)Element should form compounds with each other.
- d)Elements should have the same, valency.
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not a Hume-Ruthery condition?
a)
Crystal structure of each element of solid solution must be the same.
b)
Size of atoms of each two elements must not differ by more than 15%.
c)
Element should form compounds with each other.
d)
Elements should have the same, valency.
|
|
Akshat Mehta answered |
Crystal structure consistency:
- The crystal structure of each element in a solid solution must be the same to ensure they can form a homogeneous mixture at the atomic level.
Atomic size similarity:
- The size of atoms of each two elements in a solid solution should not differ by more than 15%. This is important to prevent distortion in the crystal lattice structure.
Valency matching:
- Elements in a solid solution should ideally have the same valency. This ensures that the atoms can effectively substitute for each other in the crystal lattice without disrupting the overall structure.
Formation of compounds:
- This condition is not a Hume-Rothery condition. While it can be advantageous for elements in a solid solution to form compounds with each other, it is not a fundamental requirement for the formation of a solid solution. The other three conditions mentioned (crystal structure consistency, atomic size similarity, and valency matching) are crucial for the formation of a stable and uniform solid solution.
- The crystal structure of each element in a solid solution must be the same to ensure they can form a homogeneous mixture at the atomic level.
Atomic size similarity:
- The size of atoms of each two elements in a solid solution should not differ by more than 15%. This is important to prevent distortion in the crystal lattice structure.
Valency matching:
- Elements in a solid solution should ideally have the same valency. This ensures that the atoms can effectively substitute for each other in the crystal lattice without disrupting the overall structure.
Formation of compounds:
- This condition is not a Hume-Rothery condition. While it can be advantageous for elements in a solid solution to form compounds with each other, it is not a fundamental requirement for the formation of a solid solution. The other three conditions mentioned (crystal structure consistency, atomic size similarity, and valency matching) are crucial for the formation of a stable and uniform solid solution.
Ms for Fe-C system is around- a)7250C
- b)5500C
- c)4500C
- d)2100C
Correct answer is option 'D'. Can you explain this answer?
Ms for Fe-C system is around
a)
7250C
b)
5500C
c)
4500C
d)
2100C

|
Rohan Singh answered |
Ms temperature: The temperature at which a martensitic transformation starts during cooling after austenitization. This temperature is around 2100C.
Austenite is a solid solution of carbon in - a)α - iron
- b)β - iron
- c)γ - iron
- d)δ - iron
Correct answer is option 'C'. Can you explain this answer?
Austenite is a solid solution of carbon in
a)
α - iron
b)
β - iron
c)
γ - iron
d)
δ - iron

|
Anshul Chakraborty answered |
Explanation:
Austenite as a solid solution of carbon in gamma iron:
- Austenite is a solid solution of carbon in gamma iron, which is a face-centered cubic (FCC) crystal structure.
- At high temperatures, above 912°C, iron exists in the gamma phase, also known as austenite.
- Austenite has a high solubility for carbon, allowing it to dissolve more carbon compared to other phases of iron.
- The carbon atoms occupy the interstitial sites within the FCC structure of austenite, resulting in the formation of a solid solution.
- This solid solution of carbon in gamma iron (austenite) is important in the heat treatment of steels, as it influences the mechanical properties and microstructure of the material.
- During the cooling process, austenite can transform into different phases such as pearlite, martensite, or bainite, depending on the cooling rate and composition of the steel.
- The presence of carbon in the austenite phase plays a significant role in determining the final properties of the steel, such as hardness, strength, and toughness.
Therefore, austenite as a solid solution of carbon in gamma iron is a crucial phase in the metallurgy of steels and is essential for achieving desired material characteristics.
Austenite as a solid solution of carbon in gamma iron:
- Austenite is a solid solution of carbon in gamma iron, which is a face-centered cubic (FCC) crystal structure.
- At high temperatures, above 912°C, iron exists in the gamma phase, also known as austenite.
- Austenite has a high solubility for carbon, allowing it to dissolve more carbon compared to other phases of iron.
- The carbon atoms occupy the interstitial sites within the FCC structure of austenite, resulting in the formation of a solid solution.
- This solid solution of carbon in gamma iron (austenite) is important in the heat treatment of steels, as it influences the mechanical properties and microstructure of the material.
- During the cooling process, austenite can transform into different phases such as pearlite, martensite, or bainite, depending on the cooling rate and composition of the steel.
- The presence of carbon in the austenite phase plays a significant role in determining the final properties of the steel, such as hardness, strength, and toughness.
Therefore, austenite as a solid solution of carbon in gamma iron is a crucial phase in the metallurgy of steels and is essential for achieving desired material characteristics.
Chapter doubts & questions for Phase Diagrams - Engineering Materials 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Phase Diagrams - Engineering Materials in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Engineering Materials
15 videos|35 docs|13 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup