All Exams >
NEET >
NCERTs at Fingertips: Textbooks, Tests & Solutions >
All Questions
All questions of Classification of Elements & Periodicity in Properties for NEET Exam
Among the elements with atomic numbers 9, 12, 16 and 36 which is highly electropositive?- a)Element with atomic number 9
- b)Element \vith atomic number 12
- c)Element with atomic number 16
- d)Element with atomic number 36
Correct answer is option 'B'. Can you explain this answer?
Among the elements with atomic numbers 9, 12, 16 and 36 which is highly electropositive?
a)
Element with atomic number 9
b)
Element \vith atomic number 12
c)
Element with atomic number 16
d)
Element with atomic number 36
|
|
Pooja Deshpande answered |
Introduction:
In order to determine which element is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36, we need to understand the concept of electronegativity and how it relates to electropositivity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Electropositivity, on the other hand, is the tendency of an atom to lose electrons and form positive ions. Therefore, an element with low electronegativity and high electropositivity will readily lose electrons and form positive ions.
Explanation:
Let's analyze each element and its electropositivity based on their atomic numbers:
Element with atomic number 9 (Fluorine):
Fluorine is a halogen and has a high electronegativity. It readily gains an electron to achieve a stable electron configuration. Therefore, it is not highly electropositive.
Element with atomic number 12 (Magnesium):
Magnesium is an alkaline earth metal and has a low electronegativity. It readily loses its two valence electrons to achieve a stable electron configuration. Therefore, it is highly electropositive.
Element with atomic number 16 (Sulfur):
Sulfur is a nonmetal and has a moderate electronegativity. It can gain or lose electrons depending on the reaction conditions. However, it is not highly electropositive as it tends to gain electrons to achieve a stable electron configuration.
Element with atomic number 36 (Krypton):
Krypton is a noble gas and has a very high electronegativity. It has a stable electron configuration and tends not to lose or gain electrons. Therefore, it is not highly electropositive.
Conclusion:
Based on the analysis, the element with atomic number 12 (Magnesium) is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36. It has a low electronegativity and readily loses its two valence electrons to form a stable 2+ ion.
In order to determine which element is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36, we need to understand the concept of electronegativity and how it relates to electropositivity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Electropositivity, on the other hand, is the tendency of an atom to lose electrons and form positive ions. Therefore, an element with low electronegativity and high electropositivity will readily lose electrons and form positive ions.
Explanation:
Let's analyze each element and its electropositivity based on their atomic numbers:
Element with atomic number 9 (Fluorine):
Fluorine is a halogen and has a high electronegativity. It readily gains an electron to achieve a stable electron configuration. Therefore, it is not highly electropositive.
Element with atomic number 12 (Magnesium):
Magnesium is an alkaline earth metal and has a low electronegativity. It readily loses its two valence electrons to achieve a stable electron configuration. Therefore, it is highly electropositive.
Element with atomic number 16 (Sulfur):
Sulfur is a nonmetal and has a moderate electronegativity. It can gain or lose electrons depending on the reaction conditions. However, it is not highly electropositive as it tends to gain electrons to achieve a stable electron configuration.
Element with atomic number 36 (Krypton):
Krypton is a noble gas and has a very high electronegativity. It has a stable electron configuration and tends not to lose or gain electrons. Therefore, it is not highly electropositive.
Conclusion:
Based on the analysis, the element with atomic number 12 (Magnesium) is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36. It has a low electronegativity and readily loses its two valence electrons to form a stable 2+ ion.
K+ and Cl- ions are isoelectronic. Which of the statements is not correct?
- a)Both K+ and Cl- ions contain 18 electrons
- b)Both K+ and Cl- ions have same configuration
- c)K+ ion is bigger than Cl- ion in ionic size
- d)Cl- ion is bigger than K+ ion in size
Correct answer is option 'C'. Can you explain this answer?
K+ and Cl- ions are isoelectronic. Which of the statements is not correct?
a)
Both K+ and Cl- ions contain 18 electrons
b)
Both K+ and Cl- ions have same configuration
c)
K+ ion is bigger than Cl- ion in ionic size
d)
Cl- ion is bigger than K+ ion in size
|
|
Ajay Yadav answered |

Which of the following elements will have highest ionisation energy?- a)1s2 2s2 2p6 3s1
- b)1s2 2s2 2p6 3s2 3p3
- c)1s2 2s2 2p6 3s2 3p4
- d)1s2 2s2 2p6 3s2 3p1
Correct answer is option 'B'. Can you explain this answer?
Which of the following elements will have highest ionisation energy?
a)
1s2 2s2 2p6 3s1
b)
1s2 2s2 2p6 3s2 3p3
c)
1s2 2s2 2p6 3s2 3p4
d)
1s2 2s2 2p6 3s2 3p1
|
|
Jaspreet answered |
In half filled and fully filled orbitals it's difficult to remove an electron from its outermost shell cause it's Highly stable electronic configuration so it's option B !!!
An element X has atomic number 19. What will be the formula of its oxide?- a)X2O
- b)XO
- c)XO2
- d)X2O3
Correct answer is option 'A'. Can you explain this answer?
An element X has atomic number 19. What will be the formula of its oxide?
a)
X2O
b)
XO
c)
XO2
d)
X2O3
|
|
Mira Joshi answered |
Element X has atomic number 19. Its valency will be one. Hence, the formula ofits oxide will be X2O.
Z = 19; 1s22s22p63s23p64s1
Z = 19; 1s22s22p63s23p64s1
Choose the incorrect statement.- a)Chemical reactivity tends to be high in group 1 metals, lower in elements in middle and increases to maximum in the group 17
- b)Halogens have very high negative electron gain enthalpy
- c)Noble gases have large positive electron gain enthalpy
- d)Decrease in electronegativities across a period is accompanied by an increase in non-metallic properties
Correct answer is option 'D'. Can you explain this answer?
Choose the incorrect statement.
a)
Chemical reactivity tends to be high in group 1 metals, lower in elements in middle and increases to maximum in the group 17
b)
Halogens have very high negative electron gain enthalpy
c)
Noble gases have large positive electron gain enthalpy
d)
Decrease in electronegativities across a period is accompanied by an increase in non-metallic properties
|
|
Palak Singh answered |
Incorrect Statement Explanation:
Decrease in electronegativities across a period is accompanied by an increase in non-metallic properties:
- This statement is incorrect because the decrease in electronegativities across a period is actually accompanied by an increase in metallic properties, not non-metallic properties.
- Electronegativity is the tendency of an atom to attract a shared pair of electrons towards itself in a chemical bond.
- As we move across a period from left to right, electronegativity generally increases. This means that elements on the left side of the periodic table (metals) have lower electronegativities compared to elements on the right side (non-metals).
- Metals tend to lose electrons and form cations, while non-metals tend to gain electrons and form anions. Therefore, a decrease in electronegativity across a period is associated with an increase in metallic properties, not non-metallic properties.
In conclusion, the statement that a decrease in electronegativities across a period is accompanied by an increase in non-metallic properties is incorrect.
The first periodic law stated by Mendeleev was:- a)There is no correlation in the properties and atomic weights of the elements
- b)The properties of the elements are a periodic function of their atomic numbers
- c)The properties of the elements are a periodic function of their atomic weights
- d)The properties of the elements are a periodic function of their empirical formula
Correct answer is option 'C'. Can you explain this answer?
The first periodic law stated by Mendeleev was:
a)
There is no correlation in the properties and atomic weights of the elements
b)
The properties of the elements are a periodic function of their atomic numbers
c)
The properties of the elements are a periodic function of their atomic weights
d)
The properties of the elements are a periodic function of their empirical formula
|
|
Dev Patel answered |
The first periodic law stated by Mendeleev was, "the physical and chemical properties of the elements are a periodic function of their atomic weights (atomic masses)." The elements having similar properties appeared in the same group (vertical column).
In the periodic table, the maximum chemical reactivity is at the extreme left (alkali metals) and extreme right (halogens). Which properties of these two groups are responsible for this?- a)Least ionisation enthalpy on the left and highest negative electron gain enthalpy on the right
- b)Non-metallic character on the left and metallic character on the right
- c)High atomic radii on the left and small atomic radii on the right
- d)Highest electronegativity on the left and least electronegativity oh the right
Correct answer is option 'A'. Can you explain this answer?
In the periodic table, the maximum chemical reactivity is at the extreme left (alkali metals) and extreme right (halogens). Which properties of these two groups are responsible for this?
a)
Least ionisation enthalpy on the left and highest negative electron gain enthalpy on the right
b)
Non-metallic character on the left and metallic character on the right
c)
High atomic radii on the left and small atomic radii on the right
d)
Highest electronegativity on the left and least electronegativity oh the right
|
|
Riya Banerjee answered |
Elements on the left side have lowest ionisation enthalpy due to which they can very easily lose electrons while the elements onthe right can accept electrons easily as they show highest negative electron gain enthalpy.
Which of the following is not a merit of Mendeleev's periodic table?- a)It helped in correcting the atomic masses of some of the elements
- b)He predicted the properties of some undiscovered elements and left gaps for them
- c)He framed the periodic table with vertical and horizontal columns and gave shape to it
- d)He gave separate places to isotopes in his periodic table
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not a merit of Mendeleev's periodic table?
a)
It helped in correcting the atomic masses of some of the elements
b)
He predicted the properties of some undiscovered elements and left gaps for them
c)
He framed the periodic table with vertical and horizontal columns and gave shape to it
d)
He gave separate places to isotopes in his periodic table
|
|
Gaurav Kumar answered |
Mendeleev could not give separate places to isotopes which had different atomic weights in the periodic table
Which is the most non-metallic element among the following?- a)1s22s22p63s1
- b)1s22s22p5
- c)1s22s22p63s2
- d)1s22s22p3
Correct answer is option 'B'. Can you explain this answer?
Which is the most non-metallic element among the following?
a)
1s22s22p63s1
b)
1s22s22p5
c)
1s22s22p63s2
d)
1s22s22p3
|
|
Janani Chopra answered |
The most non-metallic element among the options given is option 'B' with the electron configuration 1s22s22p5. Let's understand why this is the correct answer.
Understanding Metallic and Non-metallic Elements:
- Metals are elements that tend to lose electrons and form positive ions.
- Non-metals, on the other hand, tend to gain or share electrons and form negative ions or covalent bonds.
Electron Configuration:
The electron configuration of an element describes how its electrons are distributed into different energy levels and orbitals.
Analyzing the Options:
a) 1s22s22p63s1
- This electron configuration belongs to sodium (Na), which is a metal. Sodium readily loses its 3s1 electron to form a sodium ion (Na+). Therefore, it is a metallic element.
b) 1s22s22p5
- This electron configuration belongs to fluorine (F), which is a non-metal. Fluorine readily accepts an electron to complete its p orbital and form a fluoride ion (F-). Therefore, it is the most non-metallic element among the options.
c) 1s22s22p63s2
- This electron configuration belongs to magnesium (Mg), which is a metal. Magnesium readily loses its 3s2 electrons to form a magnesium ion (Mg2+). Therefore, it is a metallic element.
d) 1s22s22p3
- This electron configuration belongs to phosphorus (P), which is a non-metal. Phosphorus tends to gain three electrons to complete its p orbital and form a phosphide ion (P3-). Therefore, it is a non-metallic element.
Conclusion:
Among the given options, option 'B' with the electron configuration 1s22s22p5 represents fluorine (F), which is the most non-metallic element. Fluorine readily accepts an electron to achieve a stable electron configuration, making it highly non-metallic in nature.
Understanding Metallic and Non-metallic Elements:
- Metals are elements that tend to lose electrons and form positive ions.
- Non-metals, on the other hand, tend to gain or share electrons and form negative ions or covalent bonds.
Electron Configuration:
The electron configuration of an element describes how its electrons are distributed into different energy levels and orbitals.
Analyzing the Options:
a) 1s22s22p63s1
- This electron configuration belongs to sodium (Na), which is a metal. Sodium readily loses its 3s1 electron to form a sodium ion (Na+). Therefore, it is a metallic element.
b) 1s22s22p5
- This electron configuration belongs to fluorine (F), which is a non-metal. Fluorine readily accepts an electron to complete its p orbital and form a fluoride ion (F-). Therefore, it is the most non-metallic element among the options.
c) 1s22s22p63s2
- This electron configuration belongs to magnesium (Mg), which is a metal. Magnesium readily loses its 3s2 electrons to form a magnesium ion (Mg2+). Therefore, it is a metallic element.
d) 1s22s22p3
- This electron configuration belongs to phosphorus (P), which is a non-metal. Phosphorus tends to gain three electrons to complete its p orbital and form a phosphide ion (P3-). Therefore, it is a non-metallic element.
Conclusion:
Among the given options, option 'B' with the electron configuration 1s22s22p5 represents fluorine (F), which is the most non-metallic element. Fluorine readily accepts an electron to achieve a stable electron configuration, making it highly non-metallic in nature.
Atomic numbers of few elements are given below. Which of the pairs belongs to s-block?- a)7, 14
- b)3, 20
- c)8, 15
- d)9, 17
Correct answer is option 'B'. Can you explain this answer?
Atomic numbers of few elements are given below. Which of the pairs belongs to s-block?
a)
7, 14
b)
3, 20
c)
8, 15
d)
9, 17
|
|
Suresh Iyer answered |
Z = 3; 1s2 2s1 ; Z = 20; 1s2 2s2 2p6 3s2 3p6 4s2
Which of the following have the same number of electrons in outermost shell?- a)Elements with atomic numbers 30,48, 80
- b)Elements with atomic numbers 14, 15,16
- c)Elements with atomic numbers 20, 30, 50
- d)Elements with atomi numbers 10, 18, 26
Correct answer is option 'A'. Can you explain this answer?
Which of the following have the same number of electrons in outermost shell?
a)
Elements with atomic numbers 30,48, 80
b)
Elements with atomic numbers 14, 15,16
c)
Elements with atomic numbers 20, 30, 50
d)
Elements with atomi numbers 10, 18, 26
|
|
Ananya Das answered |
Element with atomic number 30 ; [Ar] 3d10 4s2
Element with atomic number 48 ; [Kr] 4d10 5s2
Element with atomic number 80; [Xe] 4f145d106s2
Element with atomic number 48 ; [Kr] 4d10 5s2
Element with atomic number 80; [Xe] 4f145d106s2
Given below are the names of few elements based on their position in the periodic table. Identify the element which is not correctly placed.- a)An element which tends to lose three electrons - Aluminium
- b)An element which tends to gain two electrons - Iodine
- c)An element with valency four - Silicon
- d)A transuranium element - Plutonium
Correct answer is option 'B'. Can you explain this answer?
Given below are the names of few elements based on their position in the periodic table. Identify the element which is not correctly placed.
a)
An element which tends to lose three electrons - Aluminium
b)
An element which tends to gain two electrons - Iodine
c)
An element with valency four - Silicon
d)
A transuranium element - Plutonium
|
|
Jyoti Sengupta answered |
Iodine can gain only one electron.
I + e- → I-
I + e- → I-
Indicate the wrong statement on the basis of the periodic table.- a)The most electronegative element in the periodic table is fluorine
- b)Scandium is the first transition element and belongs to fourth period
- c)There are four transition series in the periodic table each containing 10 elements
- d)Along a period halogens have maximum negative electron gain enthalphy
Correct answer is option 'C'. Can you explain this answer?
Indicate the wrong statement on the basis of the periodic table.
a)
The most electronegative element in the periodic table is fluorine
b)
Scandium is the first transition element and belongs to fourth period
c)
There are four transition series in the periodic table each containing 10 elements
d)
Along a period halogens have maximum negative electron gain enthalphy
|
|
Deepak Sharma answered |
The wrong statement on the basis of the periodic table is option C, which states that there are four transition series in the periodic table, each containing 10 elements.
Explanation:
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. It is divided into several periods (rows) and groups (columns). Each period represents the energy level or shell occupied by the outermost electrons, while each group represents elements with similar properties due to their similar valence electron configurations.
The transition elements or transition metals are a group of elements that occupy a central block in the periodic table, specifically in groups 3 to 12. They are characterized by the presence of partially filled d orbitals in their electron configurations. The transition metals have similar chemical properties, including the ability to form complex ions, exhibit variable oxidation states, and act as good catalysts.
The correct properties and characteristics related to the periodic table are:
a) The most electronegative element in the periodic table is fluorine:
Fluorine, with an atomic number of 9, is located in group 17 (group VIIA) of the periodic table. It is the most electronegative element because it has the highest tendency to attract electrons towards itself when bonded to other elements. This high electronegativity is due to its small atomic size and high effective nuclear charge.
b) Scandium is the first transition element and belongs to the fourth period:
Scandium, with an atomic number of 21, is the first element in the d-block or transition metals. It belongs to the fourth period (row) of the periodic table as it has four electron shells. Scandium has the electron configuration [Ar] 3d1 4s2.
d) Along a period, halogens have the maximum negative electron gain enthalpy:
Halogens, which include elements like fluorine, chlorine, bromine, iodine, and astatine, belong to group 17 (group VIIA) of the periodic table. They have high electron affinity or electron gain enthalpy, which refers to the energy change when an atom gains an electron to form a negative ion. Halogens have the greatest negative electron gain enthalpy along a period due to their high effective nuclear charge and small atomic size.
c) There are four transition series in the periodic table, each containing 10 elements:
This statement is incorrect. There are actually five transition series in the periodic table, not four. These series are based on the filling of the 3d, 4d, 5d, 6d, and 7d orbitals. Each series contains 10 elements. The first transition series includes the elements from scandium (Sc) to zinc (Zn), the second series includes the elements from yttrium (Y) to cadmium (Cd), the third series includes the elements from lanthanum (La) to mercury (Hg), the fourth series includes the elements from actinium (Ac) to copernicium (Cn), and the fifth series includes the elements from rutherfordium (Rf) onwards.
Therefore, option C is the wrong statement because there are actually five transition series in the periodic table, each containing 10 elements.
Explanation:
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. It is divided into several periods (rows) and groups (columns). Each period represents the energy level or shell occupied by the outermost electrons, while each group represents elements with similar properties due to their similar valence electron configurations.
The transition elements or transition metals are a group of elements that occupy a central block in the periodic table, specifically in groups 3 to 12. They are characterized by the presence of partially filled d orbitals in their electron configurations. The transition metals have similar chemical properties, including the ability to form complex ions, exhibit variable oxidation states, and act as good catalysts.
The correct properties and characteristics related to the periodic table are:
a) The most electronegative element in the periodic table is fluorine:
Fluorine, with an atomic number of 9, is located in group 17 (group VIIA) of the periodic table. It is the most electronegative element because it has the highest tendency to attract electrons towards itself when bonded to other elements. This high electronegativity is due to its small atomic size and high effective nuclear charge.
b) Scandium is the first transition element and belongs to the fourth period:
Scandium, with an atomic number of 21, is the first element in the d-block or transition metals. It belongs to the fourth period (row) of the periodic table as it has four electron shells. Scandium has the electron configuration [Ar] 3d1 4s2.
d) Along a period, halogens have the maximum negative electron gain enthalpy:
Halogens, which include elements like fluorine, chlorine, bromine, iodine, and astatine, belong to group 17 (group VIIA) of the periodic table. They have high electron affinity or electron gain enthalpy, which refers to the energy change when an atom gains an electron to form a negative ion. Halogens have the greatest negative electron gain enthalpy along a period due to their high effective nuclear charge and small atomic size.
c) There are four transition series in the periodic table, each containing 10 elements:
This statement is incorrect. There are actually five transition series in the periodic table, not four. These series are based on the filling of the 3d, 4d, 5d, 6d, and 7d orbitals. Each series contains 10 elements. The first transition series includes the elements from scandium (Sc) to zinc (Zn), the second series includes the elements from yttrium (Y) to cadmium (Cd), the third series includes the elements from lanthanum (La) to mercury (Hg), the fourth series includes the elements from actinium (Ac) to copernicium (Cn), and the fifth series includes the elements from rutherfordium (Rf) onwards.
Therefore, option C is the wrong statement because there are actually five transition series in the periodic table, each containing 10 elements.
Ionic radius in a group while moving down.- a)Remains same from top to bottom
- b)Decreases from top to bottom
- c)Increases from top to bottom
- d)First increases and then decreases
Correct answer is option 'C'. Can you explain this answer?
Ionic radius in a group while moving down.
a)
Remains same from top to bottom
b)
Decreases from top to bottom
c)
Increases from top to bottom
d)
First increases and then decreases
|
|
Jyoti Sengupta answered |
Ionic radius in a group increases from top to bottom since one energy shell is added with each period
A sudden large jump between the values of second and third ionization energies of an element would be associated with which ofthe following electronic configuration?- a)1s2, 2s2, 2p6, 3s1, 3p2
- b)1s2, 2s2, 2p6, 3s2, 3p1
- c)1s2, 2s2, 2p6, 3s1
- d)1s2, 2s2, 2p6, 3s2
Correct answer is option 'D'. Can you explain this answer?
A sudden large jump between the values of second and third ionization energies of an element would be associated with which ofthe following electronic configuration?
a)
1s2, 2s2, 2p6, 3s1, 3p2
b)
1s2, 2s2, 2p6, 3s2, 3p1
c)
1s2, 2s2, 2p6, 3s1
d)
1s2, 2s2, 2p6, 3s2
|
|
Aarav Shah answered |
Understanding Ionization Energies
Ionization energy refers to the energy required to remove an electron from an atom. A sudden large jump between the second and third ionization energies indicates that removing the third electron requires significantly more energy than removing the first two. This typically occurs when the electron being removed is from a more stable electron configuration.
Analysis of Electronic Configurations
Let’s analyze the provided electronic configurations:
- Option A: 1s2, 2s2, 2p6, 3s1, 3p2
- Removal of the first electron from 3p2 is relatively easy. Removal of a second electron from the same subshell remains manageable. The third ionization would not show a large jump since the remaining electrons are still in the same energy levels.
- Option B: 1s2, 2s2, 2p6, 3s2, 3p1
- Similar to option A, removing the first two electrons from 3s2 and 3p1 does not reach a stable noble gas configuration. Hence, the third ionization does not exhibit a significant jump.
- Option C: 1s2, 2s2, 2p6, 3s1
- Removing one electron from 3s1 still doesn’t lead to a substantial increase in ionization energy for subsequent removals, as there’s no stable electron configuration left behind.
- Option D: 1s2, 2s2, 2p6, 3s2
- Here, the removal of the first two electrons from 3s2 would occur easily, leading to a fully filled 2p subshell (a stable configuration). The third ionization would require removing an electron from a new, higher energy subshell (3p) which is much more stable, resulting in a significant jump in ionization energy.
Conclusion
Thus, option D represents an electronic configuration where the large jump between the second and third ionization energies can be attributed to the stability of the noble gas configuration achieved after the removal of two electrons from the 3s subshell.
Ionization energy refers to the energy required to remove an electron from an atom. A sudden large jump between the second and third ionization energies indicates that removing the third electron requires significantly more energy than removing the first two. This typically occurs when the electron being removed is from a more stable electron configuration.
Analysis of Electronic Configurations
Let’s analyze the provided electronic configurations:
- Option A: 1s2, 2s2, 2p6, 3s1, 3p2
- Removal of the first electron from 3p2 is relatively easy. Removal of a second electron from the same subshell remains manageable. The third ionization would not show a large jump since the remaining electrons are still in the same energy levels.
- Option B: 1s2, 2s2, 2p6, 3s2, 3p1
- Similar to option A, removing the first two electrons from 3s2 and 3p1 does not reach a stable noble gas configuration. Hence, the third ionization does not exhibit a significant jump.
- Option C: 1s2, 2s2, 2p6, 3s1
- Removing one electron from 3s1 still doesn’t lead to a substantial increase in ionization energy for subsequent removals, as there’s no stable electron configuration left behind.
- Option D: 1s2, 2s2, 2p6, 3s2
- Here, the removal of the first two electrons from 3s2 would occur easily, leading to a fully filled 2p subshell (a stable configuration). The third ionization would require removing an electron from a new, higher energy subshell (3p) which is much more stable, resulting in a significant jump in ionization energy.
Conclusion
Thus, option D represents an electronic configuration where the large jump between the second and third ionization energies can be attributed to the stability of the noble gas configuration achieved after the removal of two electrons from the 3s subshell.
The electronic configuration of few elements is given below. Mark the statement which is not correct about these elements.
(i) 1s22s22p63s1
(ii) 1s22s22p5
(iii) 1s22s22p6
(iv) 1s22s22p3- a)(i) is an alkali metal
- b)(iii) is a noble metal
- c)(i) and (ii) form ionic compounds
- d)(iv) has high ionisation enthalpy
Correct answer is option 'B'. Can you explain this answer?
The electronic configuration of few elements is given below. Mark the statement which is not correct about these elements.
(i) 1s22s22p63s1
(ii) 1s22s22p5
(iii) 1s22s22p6
(iv) 1s22s22p3
(i) 1s22s22p63s1
(ii) 1s22s22p5
(iii) 1s22s22p6
(iv) 1s22s22p3
a)
(i) is an alkali metal
b)
(iii) is a noble metal
c)
(i) and (ii) form ionic compounds
d)
(iv) has high ionisation enthalpy
|
|
Hansa Sharma answered |
(i) is an alkali metal. It readily loses an electron to attain a noble gas configuration.
(iii) is a noble gas. It has completed its octet.
Highly electropositive (i) and highly electronegative (ii) form ionic compounds.
(iv) has high ionization enthalpy as removal of an electron will break half-filled 2p subshell.
(iii) is a noble gas. It has completed its octet.
Highly electropositive (i) and highly electronegative (ii) form ionic compounds.
(iv) has high ionization enthalpy as removal of an electron will break half-filled 2p subshell.
Predict the formula of a compound formed by aluminium and sulphur.- a)Al2S2
- b)Al3S2
- c)Al2S3
- d)AIS
Correct answer is option 'C'. Can you explain this answer?
Predict the formula of a compound formed by aluminium and sulphur.
a)
Al2S2
b)
Al3S2
c)
Al2S3
d)
AIS
|
|
Raghav Bansal answered |
Al - 3e- → Al3+, S + 2e- → S2-
The formula for its sulphide is AI2S3.
The formula for its sulphide is AI2S3.
Part of the periodic table showing p-block is depicted below. What are the elements shown in the zig-zag boxes called? What is the nature of the elements outside this boundary on the right side of the table?
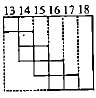
- a)Transition elements, metalloids
- b)Metalloids, non-metals
- c)Metals, non-metals
- d)Non-metals, noble gases
Correct answer is option 'B'. Can you explain this answer?
Part of the periodic table showing p-block is depicted below. What are the elements shown in the zig-zag boxes called? What is the nature of the elements outside this boundary on the right side of the table?


a)
Transition elements, metalloids
b)
Metalloids, non-metals
c)
Metals, non-metals
d)
Non-metals, noble gases
|
|
Khushi Raghuvanshi answered |
Answer 'b' is correct....
The main reason for showing anomalous properties of the first member of a group in s or p-block is- a)Maximum chemical reactivity
- b)Maximum electronegativity and different configurations
- c)Small size, large charge/radius ratio
- d)Tendency to form multiple bonds
Correct answer is option 'C'. Can you explain this answer?
The main reason for showing anomalous properties of the first member of a group in s or p-block is
a)
Maximum chemical reactivity
b)
Maximum electronegativity and different configurations
c)
Small size, large charge/radius ratio
d)
Tendency to form multiple bonds
|
|
Dev Patel answered |
Due to small size, large charge/radius ratio and high electronegativity, the first elementshows anomalous behaviour.
Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?- a)Valence principal quantum number (n)
- b)Nuclear charge (Z)
- c)Nuclear mass
- d)Number of core electrons
Correct answer is option 'C'. Can you explain this answer?
Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?
a)
Valence principal quantum number (n)
b)
Nuclear charge (Z)
c)
Nuclear mass
d)
Number of core electrons
|
|
Jyoti Sengupta answered |
Nuclear mass does not affect the valence shell because nucleus consists of protons and neutrons. Whereas proton, i.e. nuclear charge affects the valence shell but neutrons do not. Thus, option (c) is wrong.
Which of the following will have lowest electron affinity?- a)Nitrogen
- b)Oxygen
- c)Argon
- d)Boron
Correct answer is option 'C'. Can you explain this answer?
Which of the following will have lowest electron affinity?
a)
Nitrogen
b)
Oxygen
c)
Argon
d)
Boron
|
|
Vivek Patel answered |
Electron affinity of noble gases is zero.
Which is correct increasing order of their tendency of the given elements to form M3- ion?- a)Bi > Sb > As > P > N
- b)Bi < Sb < As < P < N
- c)N < P < Sb < Bi < As
- d)Bi > Sb ~ N ~ P > As
Correct answer is option 'B'. Can you explain this answer?
Which is correct increasing order of their tendency of the given elements to form M3- ion?
a)
Bi > Sb > As > P > N
b)
Bi < Sb < As < P < N
c)
N < P < Sb < Bi < As
d)
Bi > Sb ~ N ~ P > As
|
|
Gaurav Kumar answered |
On moving down the group, the stability of-3 oxidation state decreases. This is due to the following reasons (i) On descending a group the size of the atom or ion increases. As a result, attraction of the nucleus per newly added electron decreases (ii) A large anion cannot fit easily into lattice ofa small cation, (iii) As the negative charge on the ion increases, it becomes more and more susceptible to polarisation.
An element has the electronic configuration
1s2 2s2 2p6 3s2 3p6 3d8 4s2.
What will be its position in.the periodic table?- a)Period 4, Group 10
- b)Period 2, Group 2
- c)Period 4, Group 2
- d)Period 2, Group 8
Correct answer is option 'A'. Can you explain this answer?
An element has the electronic configuration
1s2 2s2 2p6 3s2 3p6 3d8 4s2.
What will be its position in.the periodic table?
1s2 2s2 2p6 3s2 3p6 3d8 4s2.
What will be its position in.the periodic table?
a)
Period 4, Group 10
b)
Period 2, Group 2
c)
Period 4, Group 2
d)
Period 2, Group 8
|
|
Hansa Sharma answered |
n = 4 hence, element lies in 4th period.
Group number = ns + (n - 1)d = 2 + 8 =10
Group number = ns + (n - 1)d = 2 + 8 =10
Which of the following is not correct statement for periodic classification of elements?- a)The properties of elements are the periodic functions of their atomic number
- b)Non-metallic elements are less in number than metallic elements
- c)For transition elements, the last electron enters into (n - 2) d-subshell
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not correct statement for periodic classification of elements?
a)
The properties of elements are the periodic functions of their atomic number
b)
Non-metallic elements are less in number than metallic elements
c)
For transition elements, the last electron enters into (n - 2) d-subshell
d)
None of these
|
|
Geetika Shah answered |
In the periodic classification of elements: The properties of elements are the periodic functions of their atomic number. Non-metallic elements are less in number than metallic elements. For transition elements, the last electron enters into (n - 1) d-subshell.
What is common between given cations and anions, O2-, F-, Na+, Mg2+, Al3+?- a)All have same ionic radii
- b)All are isoelectronic species having 10 electrons
- c)All of them belong to the third period
- d)The nature of oxides of all the ions is basic
Correct answer is option 'B'. Can you explain this answer?
What is common between given cations and anions, O2-, F-, Na+, Mg2+, Al3+?
a)
All have same ionic radii
b)
All are isoelectronic species having 10 electrons
c)
All of them belong to the third period
d)
The nature of oxides of all the ions is basic
|
|
Kavita Tripatho answered |
Because all elements have same no of electron
As we move from left to right, the electronegativity increases. An atom which is highly electronegative has- a)large size
- b)low electron affinity
- c)high ionisation enthalpy
- d)low chemical reactivity
Correct answer is option 'C'. Can you explain this answer?
As we move from left to right, the electronegativity increases. An atom which is highly electronegative has
a)
large size
b)
low electron affinity
c)
high ionisation enthalpy
d)
low chemical reactivity
|
|
Meera Singh answered |
It is difficult to remove an electron from a highly electronegative element.
What were the main demerits of Mendeleev's periodic table?
(i) Hydrogen has been placed in group I though it resembled to group VII as well.
(ii) Positions of some elements was not justified.
(iii) Isotopes were not given separate places.
(iv) Lanthanides and actinides were not included in the table.- a)(i), (ii) and (iii)
- b)(i), (ii), (iii) and (iv)
- c)(ii) and (iv)
- d)(i), (iii) and (iv)
Correct answer is option 'B'. Can you explain this answer?
What were the main demerits of Mendeleev's periodic table?
(i) Hydrogen has been placed in group I though it resembled to group VII as well.
(ii) Positions of some elements was not justified.
(iii) Isotopes were not given separate places.
(iv) Lanthanides and actinides were not included in the table.
(i) Hydrogen has been placed in group I though it resembled to group VII as well.
(ii) Positions of some elements was not justified.
(iii) Isotopes were not given separate places.
(iv) Lanthanides and actinides were not included in the table.
a)
(i), (ii) and (iii)
b)
(i), (ii), (iii) and (iv)
c)
(ii) and (iv)
d)
(i), (iii) and (iv)
|
|
Priya Menon answered |
All are the demerits of Mendeleev's periodic table.
Why is the electron gain enthalpy of O or F less than that of S or Cl?- a)O and F are more electronegative than S and Cl
- b)When an electron is added to O or F, it goes to a smaller (n = 2) level and suffers more repulsion than the electron in S or Cl in larger level (n = 3)
- c)Adding an electron to 3p-orbital leads to more repulsion than 2p-orbital
- d)Electroh gain enthalpy depends upon the electron affinity of the atom
Correct answer is option 'B'. Can you explain this answer?
Why is the electron gain enthalpy of O or F less than that of S or Cl?
a)
O and F are more electronegative than S and Cl
b)
When an electron is added to O or F, it goes to a smaller (n = 2) level and suffers more repulsion than the electron in S or Cl in larger level (n = 3)
c)
Adding an electron to 3p-orbital leads to more repulsion than 2p-orbital
d)
Electroh gain enthalpy depends upon the electron affinity of the atom
|
|
Anjali Sharma answered |
There is more repulsion for the incoming electron when the size of atom is smaller.
Law of octaves stated- a)Every eighth element had properties similar to the first element
- b)Every third element had properties similar to the first element
- c)The properties of the middle element were in between the other two members
- d)The properties of the elements were repeated after regular intervals of 3, 4 or 8 elements
Correct answer is option 'A'. Can you explain this answer?
Law of octaves stated
a)
Every eighth element had properties similar to the first element
b)
Every third element had properties similar to the first element
c)
The properties of the middle element were in between the other two members
d)
The properties of the elements were repeated after regular intervals of 3, 4 or 8 elements
|
|
Jaideep Chauhan answered |
The law of octaves is a concept proposed by John Newlands, an English chemist, in 1864. It was one of the earliest attempts to organize the elements based on their chemical properties. According to this law, when the elements are arranged in order of increasing atomic mass, the properties of every eighth element are similar to the first element.
Explanation:
1. Law of Octaves: The law of octaves states that the properties of elements are repeated after regular intervals of eight elements.
2. Arrangement of Elements: Newlands arranged the known elements in order of increasing atomic mass. He observed that when the elements were arranged in this manner, there was a periodic repetition of properties.
3. Similarity of Properties: Newlands found that every eighth element had properties that were similar to the first element. This means that the elements at the first and ninth position, second and tenth position, third and eleventh position, and so on, shared similar properties.
4. Example: For example, Newlands noticed that lithium (Li) and sodium (Na) had similar properties, so did beryllium (Be) and magnesium (Mg), boron (B) and aluminum (Al), and so on.
5. Limitations: Although the law of octaves showed periodicity in the properties of elements, it had limitations. It failed to accommodate all the known elements at that time, and it did not explain the underlying reason for the repetition of properties.
6. Recognition and Acceptance: The law of octaves was initially met with skepticism and criticism. However, it laid the foundation for the development of the modern periodic table.
7. Advancement: Dmitri Mendeleev, a Russian chemist, later expanded on Newlands' work and proposed the periodic law, which states that the properties of elements are periodic functions of their atomic numbers.
8. Modern Periodic Table: The modern periodic table is based on the periodic law and arranges the elements in order of increasing atomic number. It organizes the elements into groups and periods based on their similar properties and electronic configurations.
In conclusion, the correct answer is option 'A' - every eighth element has properties similar to the first element. This observation, known as the law of octaves, was proposed by John Newlands and served as a precursor to the development of the modern periodic table.
Explanation:
1. Law of Octaves: The law of octaves states that the properties of elements are repeated after regular intervals of eight elements.
2. Arrangement of Elements: Newlands arranged the known elements in order of increasing atomic mass. He observed that when the elements were arranged in this manner, there was a periodic repetition of properties.
3. Similarity of Properties: Newlands found that every eighth element had properties that were similar to the first element. This means that the elements at the first and ninth position, second and tenth position, third and eleventh position, and so on, shared similar properties.
4. Example: For example, Newlands noticed that lithium (Li) and sodium (Na) had similar properties, so did beryllium (Be) and magnesium (Mg), boron (B) and aluminum (Al), and so on.
5. Limitations: Although the law of octaves showed periodicity in the properties of elements, it had limitations. It failed to accommodate all the known elements at that time, and it did not explain the underlying reason for the repetition of properties.
6. Recognition and Acceptance: The law of octaves was initially met with skepticism and criticism. However, it laid the foundation for the development of the modern periodic table.
7. Advancement: Dmitri Mendeleev, a Russian chemist, later expanded on Newlands' work and proposed the periodic law, which states that the properties of elements are periodic functions of their atomic numbers.
8. Modern Periodic Table: The modern periodic table is based on the periodic law and arranges the elements in order of increasing atomic number. It organizes the elements into groups and periods based on their similar properties and electronic configurations.
In conclusion, the correct answer is option 'A' - every eighth element has properties similar to the first element. This observation, known as the law of octaves, was proposed by John Newlands and served as a precursor to the development of the modern periodic table.
The first element of the groups 1 and 2 are different from other members of the respective groups. Their behaviour is more similar to the second element of the following groups. What is this relationship known as?- a)Anomalous relationship
- b)Periodic relationship
- c)Diagonal relationship
- d)Chemical relationship
Correct answer is option 'C'. Can you explain this answer?
The first element of the groups 1 and 2 are different from other members of the respective groups. Their behaviour is more similar to the second element of the following groups. What is this relationship known as?
a)
Anomalous relationship
b)
Periodic relationship
c)
Diagonal relationship
d)
Chemical relationship
|
|
Priya Menon answered |
The relation between 1st element of a group and 2nd element of the next group is called diagonal relationship.
There are many elements in the periodic table which exhibit variable valency. This is a particular characteristic of- a)Representative elements
- b)Transition elements
- c)Noble gases
- d)Non-metals
Correct answer is option 'B'. Can you explain this answer?
There are many elements in the periodic table which exhibit variable valency. This is a particular characteristic of
a)
Representative elements
b)
Transition elements
c)
Noble gases
d)
Non-metals
|
|
Arya Bose answered |
Understanding Variable Valency
Variable valency refers to the ability of an element to exhibit different oxidation states or valencies. This characteristic is most commonly associated with transition elements.
Why Transition Elements Exhibit Variable Valency
- Presence of d-orbitals: Transition metals have partially filled d-orbitals, which allows them to lose different numbers of electrons.
- Multiple oxidation states: They can exhibit multiple oxidation states due to their ability to lose different numbers of electrons from both s and d subshells.
- Formation of complex ions: Transition elements can form complex ions, which can further allow them to display variable valencies depending on the ligands attached.
Examples of Transition Elements with Variable Valency
- Iron (Fe): Exhibits +2 and +3 states.
- Copper (Cu): Shows +1 and +2 states.
- Manganese (Mn): Can have oxidation states ranging from +2 to +7.
Distinction from Other Elements
- Representative Elements: Typically have fixed valencies based on their group number and do not exhibit the same level of variability.
- Noble Gases: Generally do not form compounds and have a complete valence shell, hence no variable valency.
- Non-metals: While some non-metals can have multiple oxidation states (like sulfur), they do not exhibit the extensive variability seen in transition metals.
In conclusion, the transition elements are unique in their ability to showcase variable valency due to their electronic configuration and involvement in complex formation. This property is essential in various chemical reactions and applications, making them a significant focus in chemistry.
Variable valency refers to the ability of an element to exhibit different oxidation states or valencies. This characteristic is most commonly associated with transition elements.
Why Transition Elements Exhibit Variable Valency
- Presence of d-orbitals: Transition metals have partially filled d-orbitals, which allows them to lose different numbers of electrons.
- Multiple oxidation states: They can exhibit multiple oxidation states due to their ability to lose different numbers of electrons from both s and d subshells.
- Formation of complex ions: Transition elements can form complex ions, which can further allow them to display variable valencies depending on the ligands attached.
Examples of Transition Elements with Variable Valency
- Iron (Fe): Exhibits +2 and +3 states.
- Copper (Cu): Shows +1 and +2 states.
- Manganese (Mn): Can have oxidation states ranging from +2 to +7.
Distinction from Other Elements
- Representative Elements: Typically have fixed valencies based on their group number and do not exhibit the same level of variability.
- Noble Gases: Generally do not form compounds and have a complete valence shell, hence no variable valency.
- Non-metals: While some non-metals can have multiple oxidation states (like sulfur), they do not exhibit the extensive variability seen in transition metals.
In conclusion, the transition elements are unique in their ability to showcase variable valency due to their electronic configuration and involvement in complex formation. This property is essential in various chemical reactions and applications, making them a significant focus in chemistry.
Few general names are given along with their valence shell configurations. Mark the incorrect name.- a)ns2 np6 - Noble gases
- b)ns2 np5 - Halogens
- c)ns1 - Alkali metals
- d)ns2 np2 - Chalcogens
Correct answer is option 'D'. Can you explain this answer?
Few general names are given along with their valence shell configurations. Mark the incorrect name.
a)
ns2 np6 - Noble gases
b)
ns2 np5 - Halogens
c)
ns1 - Alkali metals
d)
ns2 np2 - Chalcogens
|
|
Ajay Yadav answered |
ns2 np2 are members of carbon family.
Chalcogens is the general name of 16th group elements.
Chalcogens is the general name of 16th group elements.
The correct order of acidic character of oxides in third period of periodic table is
- a)SiO2 < P4O10 < SO3 < CI2O7
- b)Cl2O7 < SO3 < P4O10 < SiO2
- c)SO3 < CI2O2 < P4O10 < SiO2
- d)SiO2 < CI2O7 < P4O10 < SO3
Correct answer is option 'A'. Can you explain this answer?
The correct order of acidic character of oxides in third period of periodic table is
a)
SiO2 < P4O10 < SO3 < CI2O7
b)
Cl2O7 < SO3 < P4O10 < SiO2
c)
SO3 < CI2O2 < P4O10 < SiO2
d)
SiO2 < CI2O7 < P4O10 < SO3
|
|
Jyoti Sengupta answered |
Acidic character of oxides increases as we move from left to right in a period.
To which group,an element with atomic number 88 will belong?- a)Group 12
- b)Group 17
- c)Group 10
- d)Group 2
Correct answer is option 'D'. Can you explain this answer?
To which group,an element with atomic number 88 will belong?
a)
Group 12
b)
Group 17
c)
Group 10
d)
Group 2
|
|
Mira Joshi answered |
Z = 88, [Rn] 7s2, hence belongs to group 2.
An element has atomic number 79. Predict the group and period in which the element is placed.- a)2nd group, 7th period
- b)11th group, 6th period
- c)13th group, 6th period
- d)12th group, 6th period
Correct answer is option 'B'. Can you explain this answer?
An element has atomic number 79. Predict the group and period in which the element is placed.
a)
2nd group, 7th period
b)
11th group, 6th period
c)
13th group, 6th period
d)
12th group, 6th period
|
|
Meera Singh answered |
Z = 79 [Xe]4f145d10.6s1 (n = 6)
As n = 6, the element belongs to 6th period.
Group = ns + (n - I)d, I + 10 = 11
As n = 6, the element belongs to 6th period.
Group = ns + (n - I)d, I + 10 = 11
Which of the following elettients will have highest second ionisation enthalpy?- a)1s22s22p63s2
- b)1s22s22p63s1
- c)1s22s22p63s23p2
- d)1s22s22p63s23p3
Correct answer is option 'B'. Can you explain this answer?
Which of the following elettients will have highest second ionisation enthalpy?
a)
1s22s22p63s2
b)
1s22s22p63s1
c)
1s22s22p63s23p2
d)
1s22s22p63s23p3
|
|
Suresh Iyer answered |
After losing one electron, the atom will get a stable configuration and its second ionisation energy will be very high. Hence, option (b) is correct.
Which of the following statements regarding the variation of atomic radii in the periodic table is not true?- a)In a group, there is continuous increase in size with increase in atomic number
- b)In 4f-series, there is a continuous decrease in size with increase in atomic number
- c)The size of inert gases is larger than halogens
- d)In 3rd period, the size of atoms increases with increase in atomic number
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements regarding the variation of atomic radii in the periodic table is not true?
a)
In a group, there is continuous increase in size with increase in atomic number
b)
In 4f-series, there is a continuous decrease in size with increase in atomic number
c)
The size of inert gases is larger than halogens
d)
In 3rd period, the size of atoms increases with increase in atomic number
|
|
Shanaya Sengupta answered |
Explanation:
In the periodic table, the atomic radius refers to the size of an atom. It is defined as the distance between the nucleus and the outermost electron shell of an atom. The atomic radius varies across the periodic table due to the distribution of electrons and the effective nuclear charge experienced by the outermost electrons.
Statement (a): In a group, there is continuous increase in size with increase in atomic number
This statement is true. In a group, elements have the same number of valence electrons and similar electron configurations. As we move down a group, the number of electron shells increases, resulting in an increase in atomic size. This is due to the shielding effect of inner electron shells, which reduces the attraction between the nucleus and the outermost electrons.
Statement (b): In 4f-series, there is a continuous decrease in size with increase in atomic number
This statement is true. The 4f-series consists of the lanthanides, which are located in the f-block of the periodic table. As we move across the lanthanide series, the atomic number increases, resulting in a decrease in atomic size. This is because the increasing number of protons in the nucleus exerts a stronger attraction on the electrons, leading to a contraction in atomic radius.
Statement (c): The size of inert gases is larger than halogens
This statement is true. Inert gases, also known as noble gases, have completely filled electron shells. They are located in Group 18 of the periodic table. The halogens, on the other hand, are located in Group 17. Inert gases have larger atomic radii compared to halogens because inert gases have more electron shells and experience less effective nuclear charge due to greater electron-electron repulsion.
Statement (d): In 3rd period, the size of atoms increases with increase in atomic number
This statement is not true. In the 3rd period, the atomic size does not strictly increase with atomic number. The trend is not continuous due to the difference in electron configurations. While the atomic size generally increases from left to right across a period, there are exceptions due to variations in the effective nuclear charge and electron-electron repulsion. For example, the atomic radius of sodium (Na) is larger than that of magnesium (Mg) in the 3rd period.
Therefore, the correct answer is option D - In the 3rd period, the size of atoms increases with increase in atomic number.
In the periodic table, the atomic radius refers to the size of an atom. It is defined as the distance between the nucleus and the outermost electron shell of an atom. The atomic radius varies across the periodic table due to the distribution of electrons and the effective nuclear charge experienced by the outermost electrons.
Statement (a): In a group, there is continuous increase in size with increase in atomic number
This statement is true. In a group, elements have the same number of valence electrons and similar electron configurations. As we move down a group, the number of electron shells increases, resulting in an increase in atomic size. This is due to the shielding effect of inner electron shells, which reduces the attraction between the nucleus and the outermost electrons.
Statement (b): In 4f-series, there is a continuous decrease in size with increase in atomic number
This statement is true. The 4f-series consists of the lanthanides, which are located in the f-block of the periodic table. As we move across the lanthanide series, the atomic number increases, resulting in a decrease in atomic size. This is because the increasing number of protons in the nucleus exerts a stronger attraction on the electrons, leading to a contraction in atomic radius.
Statement (c): The size of inert gases is larger than halogens
This statement is true. Inert gases, also known as noble gases, have completely filled electron shells. They are located in Group 18 of the periodic table. The halogens, on the other hand, are located in Group 17. Inert gases have larger atomic radii compared to halogens because inert gases have more electron shells and experience less effective nuclear charge due to greater electron-electron repulsion.
Statement (d): In 3rd period, the size of atoms increases with increase in atomic number
This statement is not true. In the 3rd period, the atomic size does not strictly increase with atomic number. The trend is not continuous due to the difference in electron configurations. While the atomic size generally increases from left to right across a period, there are exceptions due to variations in the effective nuclear charge and electron-electron repulsion. For example, the atomic radius of sodium (Na) is larger than that of magnesium (Mg) in the 3rd period.
Therefore, the correct answer is option D - In the 3rd period, the size of atoms increases with increase in atomic number.
Which of the foEowing statements regarding an anion is not true?- a)The gain of an electron leads to the formation of an anion
- b)The radius of the anion is larger than the atomic radius of its parent atom
- c)The effective nuclear charge increases when an anion is formed
- d)Electron cloud expands due to increased repulsion among the electrons
Correct answer is option 'C'. Can you explain this answer?
Which of the foEowing statements regarding an anion is not true?
a)
The gain of an electron leads to the formation of an anion
b)
The radius of the anion is larger than the atomic radius of its parent atom
c)
The effective nuclear charge increases when an anion is formed
d)
Electron cloud expands due to increased repulsion among the electrons
|
|
Riya Banerjee answered |
The effective nuclear charge decreases when an anion is formed due to increase in number of electrons.
Electronic configuration of four elements is given below. Which of the following does not belong to the same group?- a)[Kr] 4d105s2
- b)[Ar] 3d10 4s2
- c)[Xe] 5p6 6s2
- d)[Xe]4f175d106s2
Correct answer is option 'C'. Can you explain this answer?
Electronic configuration of four elements is given below. Which of the following does not belong to the same group?
a)
[Kr] 4d105s2
b)
[Ar] 3d10 4s2
c)
[Xe] 5p6 6s2
d)
[Xe]4f175d106s2
|
|
Raghav Bansal answered |
It belongs to p-block while other elements belong to d-block and same group.
Which of the following is arranged in order of increasing metallic character?- a)P < Si < Na < Be < Mg
- b)Be < Mg < P < Na < Si
- c)Si < Be > Mg < Na < P
- d)P < Si < Be < Mg < Na
Correct answer is option 'D'. Can you explain this answer?
Which of the following is arranged in order of increasing metallic character?
a)
P < Si < Na < Be < Mg
b)
Be < Mg < P < Na < Si
c)
Si < Be > Mg < Na < P
d)
P < Si < Be < Mg < Na
|
|
Sinjini Yadav answered |
The order of increasing metallic character is as follows:
P
P
Which of the following sets of oxides is amphoteric in nature?- a)AI2O3, As2O3, ZnO
- b)CO, NO, N2O
- c)SO3, SO2, CI2O7
- d)Na2O, MgO, BaO
Correct answer is option 'A'. Can you explain this answer?
Which of the following sets of oxides is amphoteric in nature?
a)
AI2O3, As2O3, ZnO
b)
CO, NO, N2O
c)
SO3, SO2, CI2O7
d)
Na2O, MgO, BaO
|
|
Lavanya Menon answered |
AI2O3, As2O3 and ZnO are amphoteric in nature.
Electronic configurations of few elements are given below. Mark the incorrect match.- a)1s2 2s2 2p5 - Most electronegative element
- b)1s2 2s2 2p3 - An element belonging to 3rd period and 5th group
- c)1s2 2s2 2p6 3s2 3p6 3d8 4s2 - A d-block element
- d)1s2 2s2 2p6 3s2 3p6 - An element from 18th group
Correct answer is option 'B'. Can you explain this answer?
Electronic configurations of few elements are given below. Mark the incorrect match.
a)
1s2 2s2 2p5 - Most electronegative element
b)
1s2 2s2 2p3 - An element belonging to 3rd period and 5th group
c)
1s2 2s2 2p6 3s2 3p6 3d8 4s2 - A d-block element
d)
1s2 2s2 2p6 3s2 3p6 - An element from 18th group
|
|
Suresh Iyer answered |
Since n = 2 it belongs to 2nd period.
Examples of elements belonging to s, p, d or f-block are given below. Identify the wrong example,- a)s-block element - Caesium
- b)p-block element - Barium
- c)d-block element - Chromium
- d)f-block element - Thorium
Correct answer is option 'B'. Can you explain this answer?
Examples of elements belonging to s, p, d or f-block are given below. Identify the wrong example,
a)
s-block element - Caesium
b)
p-block element - Barium
c)
d-block element - Chromium
d)
f-block element - Thorium
|
|
Jyoti Sengupta answered |
Barium belongs to s-block with an outer configuration 6s2.
Which of the following arrangements represents the correct order of electron gain enthalpy?- a)O < S < F < Cl
- b)Cl < F < S < O
- c)S < O < Cl < F
- d)F < Cl < O < S
Correct answer is option 'A'. Can you explain this answer?
Which of the following arrangements represents the correct order of electron gain enthalpy?
a)
O < S < F < Cl
b)
Cl < F < S < O
c)
S < O < Cl < F
d)
F < Cl < O < S
|
|
Ananya Das answered |
Electron gain enthalpy becomes more negative across a period while it becomes less negative in a group.
However, electron gain enthalpy of O or F is less than that of the succeeding element. When electron is added to n = 2, the repulsion is more than when it is added to n = 3 in case of Cl or S.
However, electron gain enthalpy of O or F is less than that of the succeeding element. When electron is added to n = 2, the repulsion is more than when it is added to n = 3 in case of Cl or S.
The electronic states X and Y of an atom are depicted below:
X : 1s2 2s2 2p6 3s1
Y : 1s2 2s2 2p6 3s2 3p6 4s1
Which of the following statements is not correct?- a)X represents an alkali metal
- b)Energy is required to change X into Y
- c)Y represents ground state of the element
- d)Less energy is required to remove an electron from X than from Y
Correct answer is option 'D'. Can you explain this answer?
The electronic states X and Y of an atom are depicted below:
X : 1s2 2s2 2p6 3s1
Y : 1s2 2s2 2p6 3s2 3p6 4s1
Which of the following statements is not correct?
X : 1s2 2s2 2p6 3s1
Y : 1s2 2s2 2p6 3s2 3p6 4s1
Which of the following statements is not correct?
a)
X represents an alkali metal
b)
Energy is required to change X into Y
c)
Y represents ground state of the element
d)
Less energy is required to remove an electron from X than from Y
|
|
Preethi Mukherjee answered |
Explanation:
- The electronic states X and Y of an atom are given as follows:
X: 1s2 2s2 2p6 3s1
Y: 1s2 2s2 2p6 3s2 3p6 4s1
a) X represents an alkali metal:
- Alkali metals are the elements in Group 1 of the periodic table.
- The electronic configuration of the alkali metals in the ground state is ns1, where n is the principal quantum number.
- The electronic state X has the electronic configuration 1s2 2s2 2p6 3s1, which does not match the electronic configuration of alkali metals.
- Therefore, statement a) is not correct.
b) Energy is required to change X into Y:
- The electronic state X has only one electron in the 3s orbital, while the electronic state Y has one electron in the 4s orbital.
- To change from X to Y, energy is required to move the electron from the 3s orbital to the higher energy 4s orbital.
- Therefore, statement b) is correct.
c) Y represents the ground state of the element:
- The ground state of an atom is the electronic state with the lowest energy.
- The electronic state Y has the full electronic configuration 1s2 2s2 2p6 3s2 3p6 4s1, which is not the ground state configuration.
- Therefore, statement c) is not correct.
d) Less energy is required to remove an electron from X than from Y:
- The energy required to remove an electron from an atom is known as ionization energy.
- In general, ionization energy increases as you move across a period and decreases as you move down a group in the periodic table.
- The electronic state X has an electron in the 3s orbital, while the electronic state Y has an electron in the 4s orbital.
- Since the 4s orbital is farther from the nucleus than the 3s orbital, it is easier to remove an electron from the 4s orbital.
- Therefore, less energy is required to remove an electron from Y than from X.
- Hence, statement d) is not correct.
Conclusion:
- The correct statement among the given options is d) Less energy is required to remove an electron from X than from Y.
- The electronic states X and Y of an atom are given as follows:
X: 1s2 2s2 2p6 3s1
Y: 1s2 2s2 2p6 3s2 3p6 4s1
a) X represents an alkali metal:
- Alkali metals are the elements in Group 1 of the periodic table.
- The electronic configuration of the alkali metals in the ground state is ns1, where n is the principal quantum number.
- The electronic state X has the electronic configuration 1s2 2s2 2p6 3s1, which does not match the electronic configuration of alkali metals.
- Therefore, statement a) is not correct.
b) Energy is required to change X into Y:
- The electronic state X has only one electron in the 3s orbital, while the electronic state Y has one electron in the 4s orbital.
- To change from X to Y, energy is required to move the electron from the 3s orbital to the higher energy 4s orbital.
- Therefore, statement b) is correct.
c) Y represents the ground state of the element:
- The ground state of an atom is the electronic state with the lowest energy.
- The electronic state Y has the full electronic configuration 1s2 2s2 2p6 3s2 3p6 4s1, which is not the ground state configuration.
- Therefore, statement c) is not correct.
d) Less energy is required to remove an electron from X than from Y:
- The energy required to remove an electron from an atom is known as ionization energy.
- In general, ionization energy increases as you move across a period and decreases as you move down a group in the periodic table.
- The electronic state X has an electron in the 3s orbital, while the electronic state Y has an electron in the 4s orbital.
- Since the 4s orbital is farther from the nucleus than the 3s orbital, it is easier to remove an electron from the 4s orbital.
- Therefore, less energy is required to remove an electron from Y than from X.
- Hence, statement d) is not correct.
Conclusion:
- The correct statement among the given options is d) Less energy is required to remove an electron from X than from Y.
The periodic table of today owes its development to two chemists namely:- a)Rutherford and Moseley
- b)Alexander Newlands and Dobereiner
- c)Dmitri Mendeleev and Lothar Meyer
- d)De Broglie and Neil Bohr
Correct answer is option 'C'. Can you explain this answer?
The periodic table of today owes its development to two chemists namely:
a)
Rutherford and Moseley
b)
Alexander Newlands and Dobereiner
c)
Dmitri Mendeleev and Lothar Meyer
d)
De Broglie and Neil Bohr
|
|
Preeti Iyer answered |
The periodic table of today owes its development to two chemists namely the Russian chemist Dmitri Mendeleev and the German chemist J. Lother Meyer. In 1869, they independently proposed that when the elements are arranged in the increasing order of their atomic weights, similarities in physical and chemical properties appear at regular intervals.
An element 'P' has atomic number 56. What will be the formula of its halide?- a)PX
- b)PX2
- c)PX3
- d)P2X3
Correct answer is option 'B'. Can you explain this answer?
An element 'P' has atomic number 56. What will be the formula of its halide?
a)
PX
b)
PX2
c)
PX3
d)
P2X3
|
|
Geetika Shah answered |
Z = 56; [Xe] 6s2
Formula of halide is PX2.
Formula of halide is PX2.
Chapter doubts & questions for Classification of Elements & Periodicity in Properties - NCERTs at Fingertips: Textbooks, Tests & Solutions 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Classification of Elements & Periodicity in Properties - NCERTs at Fingertips: Textbooks, Tests & Solutions in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup